体外和体内研究表明,靶向cdk9的降解物PO-8通过抑制JAK-STAT信号通路减轻lps诱导的炎症。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
细胞周期蛋白依赖性激酶9 (CDK9)作为炎症反应的关键激活剂出现,但CDK9降解物在炎症中的治疗潜力仍未得到很大程度的探索。在这项研究中,我们证明了iCDK9,一种选择性CDK9抑制剂,可以有效地抑制脂多糖(LPS)诱导的RAW 264.7巨噬细胞炎症。利用蛋白水解靶向嵌合体(PROTAC)技术,我们设计并合成了12个基于icdk9的PROTAC分子。其中PO-8被认为是一种极好的CDK9降解物,具有很强的抗炎活性。此外,PO-8通过泛素-蛋白酶体系统选择性诱导CDK9降解,导致体外和体内lps引发的炎症反应显著减弱。值得注意的是,与亲本抑制剂iCDK9相比,PO-8表现出更高的安全性和更宽的治疗窗口。机制研究表明,po -8介导的CDK9降解破坏JAK2-STAT3信号通路,从而减轻炎症。据我们所知,这项研究首次确定了小分子CDK9降解物的抗炎功效,强调了基于protac的CDK9靶向治疗是一种很有前景的炎症性疾病治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
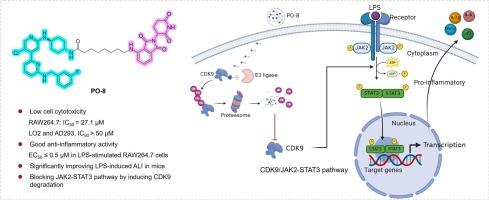
The CDK9-targeting degrader PO-8 alleviates LPS-induced inflammation by inhibiting JAK-STAT signaling in vitro and in vivo
Cyclin-dependent kinase 9 (CDK9) emerges as a crucial activator of inflammatory responses, but the therapeutic potential of CDK9 degraders in inflammation remains largely unexplored. In this study, we demonstrated that iCDK9, a selective CDK9 inhibitor, effectively suppressed lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. Leveraging proteolysis-targeting chimera (PROTAC) technology, we designed and synthesized twelve iCDK9-based PROTAC molecules. Among them, PO-8 was identified as an excellent CDK9 degrader with potent anti-inflammatory activity. Moreover, PO-8 selectively induced CDK9 degradation via the ubiquitin–proteasome system, resulting in a significant attenuation of LPS-triggered inflammatory responses both in vitro and in vivo. Notably, PO-8 exhibited an improved safety profile and a wider therapeutic window compared to its parent inhibitor, iCDK9. Mechanistic studies revealed that PO-8-mediated CDK9 degradation disrupts the JAK2-STAT3 signaling pathway, thereby mitigating inflammation. To the best of our knowledge, this study is the first to establish the anti-inflammatory efficacy of a small-molecule CDK9 degrader, highlighting PROTAC-based CDK9 targeting as a promising therapeutic strategy for inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


