G蛋白偶联雌激素受体对人内皮细胞硫化氢生成及半胱硫氨酸γ-裂解酶表达的影响。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
硫化氢(H2S)和一氧化氮(NO)是心血管稳态的重要调节因子。在内皮细胞中,H2S主要由半胱甘氨酸γ-裂解酶(CSE)合成,以l -半胱氨酸和同型半胱氨酸为底物。然而,尚不清楚G蛋白偶联雌激素受体1 (GPER)如何影响内皮细胞中H2S的产生。在这项研究中,我们研究了GPER激活是否通过上调内皮细胞中CSE的表达来促进H2S的产生,并确定了潜在的分子机制。G-1增加了cAMP反应元件结合(CREB)、蛋白激酶B (Akt)、细胞外信号调节激酶1/2 (ERK1/2)、amp活化蛋白激酶(AMPK)、Ca2+/钙调素蛋白激酶β (CaMKKβ)和Ca2+/钙调素依赖性蛋白激酶IIα (CaMKIIα)的磷酸化。阻断Akt、ERK1/2、AMPK、CaMKKβ和CaMKIIα信号通路可通过抑制CREB转录活性抑制g -1诱导的CSE表达。此外,通过抑制GPER亚基G蛋白αq亚基(Gαq)和G蛋白βγ亚基复合物(Gβγ),可以抑制CSE的表达。特别是G-1诱导人内皮EA.hy926细胞CSE表达,增加H2S生成,抑制肿瘤坏死因子α (TNF-α)诱导的核因子κ B (NF-kB)表达,降低细胞内粘附分子的表达。这些发现证实,GPER通过新的信号机制调节CSE表达影响H2S的产生,这可以作为预防心血管疾病内皮功能障碍的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
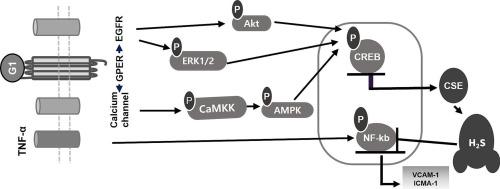
Effects of G protein-coupled estrogen receptor on hydrogen sulfide production and cystathionine γ-lyase expression in human endothelial cells
Hydrogen sulfide (H2S) is an important regulator of cardiovascular homeostasis, along with nitric oxide (NO). In endothelial cells, H2S is primarily synthesized by cystathionine γ-lyase (CSE) using L-cysteine and homocysteine as substrates. However, it has not been elucidated how G protein-coupled estrogen receptor 1 (GPER) affects H2S production in endothelial cells. In this study, we investigated whether GPER activation enhances H2S production by upregulating CSE expression in endothelial cells, and identified the underlying molecular mechanism. G-1 increased the phosphorylation of cAMP response element binding (CREB), protein kinase B (Akt), extracellular signal-regulated kinase 1/2 (ERK1/2), AMP-activated protein kinase (AMPK), Ca2+/calmodulin protein kinase kinase β (CaMKKβ), and Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα). Blockade of Akt, ERK1/2, AMPK, CaMKKβ, and CaMKIIα signaling pathways suppressed G-1-induced CSE expression by inhibiting CREB transcriptional activity. In addition, CSE expression was suppressed by inhibiting G protein alpha q subunit (Gαq) and G protein beta gamma subunit complex (Gβγ), which are subunits of GPER. In particular, G-1 induced CSE expression and increased H2S production in human endothelial EA.hy926 cells, inhibited tumor necrosis factor-alpha (TNF-α)-induced expression of nuclear factor kappa B (NF-kB), and decreased the expression of intracellular adhesion molecules. These findings confirm that GPER affects H2S production by regulating CSE expression through novel signaling mechanisms, which could be utilized as a potential therapeutic target to prevent endothelial dysfunction in cardiovascular disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


