超越免疫:rig - 1在非传染性疾病中作为细胞命运的双功能调节剂。
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
视黄酸诱导基因(Retinoic acid-inducible gene, RIG-I)因识别5'端带有三磷酸基序的短链RNA参与抗病毒先天免疫应答而受到高度重视。最近的研究表明,在RNA病毒感染的终末阶段,rig - 1的表达仍然上调,即使在干扰素产生终止后也会持续。此外,研究已经记录了RIG-I在非病毒性疾病背景下表达的上调或激活,这意味着RIG-I可能具有非免疫作用。rig - 1具有多个结构域,包括Cards、解旋酶和CTD,这些结构域赋予其在响应不同刺激时的功能多样性。这些结构域使rig - 1能够参与病毒免疫之外的各种生物过程,如程序性细胞死亡(PCD)、肿瘤免疫和细胞增殖。本文综述了rig - 1在PCD调控中的作用,包括细胞凋亡、免疫原性细胞死亡、坏死坏死、焦亡和自噬。我们系统地分析了RIG-I调节这些PCD通路的分子机制,并讨论了RIG-I靶向激活剂和拮抗剂的治疗潜力。这些进展为开发与rig - 1失调相关疾病的新疗法提供了重要见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
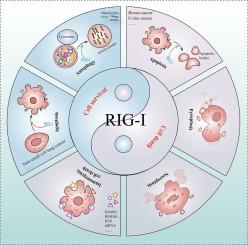
Beyond Immunity: RIG-I as a Bifunctional Regulator of Cell Fate in Non-Infectious Diseases
Retinoic acid-inducible gene (RIG-I) is highly regarded for recognizing short-stranded RNA with a triphosphate motif at the 5′ end to participate in the antiviral innate immune response. Recent studies have demonstrated that RIG-I expression remains upregulated during the terminal phase of RNA virus infection, persisting even after interferon production has terminated. Furthermore, studies have demonstrated the up-regulation or activation of RIG-I expression in non-viral disease contexts, implying a potential non-immune role for RIG-I. RIG-I possesses multiple structural domains, including Cards, Helicase, and CTD, which confer functional versatility in response to diverse stimuli. These domains enable RIG-I to participate in various biological processes beyond viral immunity, such as programmed cell death (PCD), tumor immunity, and cell proliferation. This review focuses on the role of RIG-I in regulating PCD, encompassing apoptosis, immunogenic cell death, necroptosis, pyroptosis, and autophagy. We systematically analyze the molecular mechanisms by which RIG-I modulates these PCD pathways and discuss the therapeutic potential of RIG-I-targeting activators and antagonists. These advances provide critical insights for developing novel treatments for diseases linked to RIG-I dysregulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


