纳米流体的热导率与其结构、电子和热力学性质的相关性:DFT研究
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在这项研究中,我们解决了提高纳米流体导热性的关键挑战,这是提高各种工业应用中传热效率的关键因素。利用密度泛函理论(DFT),我们系统地研究了银(Ag)、铜(Cu)、金(Au)和铝(Al)纳米流体的导热性与结构、电子和热力学性质之间的关系。我们分析了量子化学参数,包括分子轨道能量、能隙(ΔEGAP)、电离势(I)、电子亲和力(A)、硬度(η)和局部柔软度(σ),以及热力学性质,如焓(ΔH)、吉布斯自由能(ΔG)、熵(ΔS)和热容(Cv)。结果表明,Ag纳米流体具有优异的导热性能(429 W/m·K),其较高的EHOMO(−3.5325 eV)、ELUMO(−2.0781 eV)和σ (1.3751 eV),以及较低的ΔEGAP (1.4544 eV)、I (3.5325 eV)、A (2.0781 eV)和η (0.7272 eV)。这些发现揭示了热导率和量子化学参数之间的强相关性。此外,Ag和Al纳米流体的热力学计算强调,Ag的高导热性是由其升高的ΔH、ΔS和Cv值驱动的。这项工作通过建立导热系数和基本材料特性之间的定量联系,为高性能纳米流体的设计提供了新的见解,超越了以往的文献研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
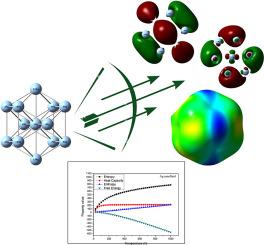
Correlation between thermal conductivity of nanofluids and their structural, electronic and thermodynamic properties: DFT study
In this study, we address the critical challenge of enhancing thermal conductivity in nanofluids, a key factor for improving heat transfer efficiency in various industrial applications. Using density functional theory (DFT), we systematically investigate the relationship between thermal conductivity and the structural, electronic, and thermodynamic properties of Silver (Ag), Copper (Cu), Gold (Au), and Aluminum (Al) nanofluids. We analyze quantum chemical parameters, including molecular orbital energies, energy gap (ΔEGAP), ionization potential (I), electron affinity (A), hardness (η), and local softness (σ), alongside thermodynamic properties such as enthalpy (ΔH), Gibbs free energy (ΔG), entropy (ΔS), and heat capacity (Cv). Our results demonstrate that Ag nanofluids exhibit superior thermal conductivity (429 W/m·K), supported by their high values of EHOMO (−3.5325 eV), ELUMO (−2.0781 eV), and σ (1.3751), as well as low values of ΔEGAP (1.4544 eV), I (3.5325 eV), A (2.0781 eV), and η (0.7272 eV). These findings reveal a strong correlation between thermal conductivity and quantum chemical parameters. Additionally, thermodynamic calculations for Ag and Al nanofluids highlight that the high thermal conductivity of Ag is driven by its elevated ΔH, ΔS, and Cv values. This work provides novel insights into the design of high-performance nanofluids by establishing a quantitative link between thermal conductivity and fundamental material properties, advancing beyond previous studies in the literature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


