超级电容器用聚苯胺电极纳米结构氧化铈的设计与合成
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
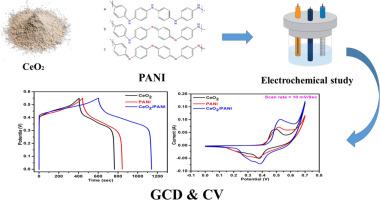
目前科学研究的重点是克服能量储存问题。为此,选择了具有高性能和长期稳定性的超级电容器(SC)。SC的主要部分是它的电极。过渡金属氧化物(TMOs)因其高比电容(Cs)和低成本而成为极具吸引力的SC电极。由于单一金属氧化物的表面积较小,为了克服这一问题,我们采用快速有效的单步超声技术制备了CeO2/PANI纳米复合材料。利用x射线衍射(XRD)等物理表征方法分析了制备材料的结构性质,并利用扫描电子显微镜(SEM)检测了制备材料的形貌,特别是CeO2/PANI纳米复合材料。CeO2/PANI纳米复合材料在1 A/g时的电化学性能表现出优异的c1076.05 F/g和Ed (36.47 Wh/kg)。此外,CeO2/PANI表现出优异的电化学性能,在未来具有很大的创新潜力。目前的研究表明,聚苯胺(PANI)和CeO2的组合提高了储能效率,并表明它可以在未来的SCs中得到应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design and synthesis of nanostructured cerium oxide anchored on polyaniline electrode for supercapacitors
The current focus of scientific study is to overcome energy storage problems. For this purpose, supercapacitor (SC) was choosen due to its high performance and long stability. The main part of SC is its electrodes. Transition metal oxides (TMOs) are attractive SC electrodes due to their high specific capacitance (Cs) and low cost. While single metal oxide shows smaller surface area, to overcome this issue we fabricate CeO2/PANI nanocomposite by employing a quick and effective single-step sonification technique. Physical characterization like X-ray diffraction (XRD) was used to analyze structural nature and scanning electron microscopy (SEM) to examine morphology of manufactured materials, specifically CeO2/PANI nanocomposite. Electrochemical properties of the nanocomposite CeO2/PANI showed outstanding Cs 1076.05 F/g and Ed (36.47 Wh/kg) at 1 A/g. Furthermore, CeO2/PANI displays excellent electrochemical capacities with great potential for innovation in the future. The present research shows that composition of polyaniline (PANI) and CeO2 increases energy storage efficiency and indicates that it can be utilized in SCs in future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


