由中心到轴向手性诱导的空间阻碍立体化学络合BINOL衍生物的反选择合成
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
提出了一种序贯策略,用于具有多个立体元素和多达四个立体中心的立体受阻BINOL衍生物的新家族的atroo选择性构建。该序列开始于一个有机催化的区域和对映选择性单二氢苯并呋喃环,建立两个立体碳原子,然后是高度选择性的铜催化的有氧氧化同源或交叉偶联,由于几乎完美的手性诱导,固定了轴向手性。此外,所得到的BINOL衍生物可以作为前体,用于通过轴向向中心手性转化合成复杂的螺杂环,通过轴向向螺旋转化合成类杂螺旋分子,或通过1,2-芳基迁移合成atrop异构萘[2,1-b]呋喃支架,进一步强调了这种基于实际的中心向轴向手性诱导的新的atropselective策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
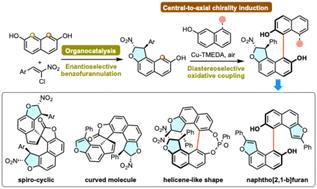
Atroposelective synthesis of sterically hindered stereochemically complex BINOL derivatives via central-to-axial chirality induction
A sequential strategy is proposed for the atroposelective construction of new families of sterically hindered BINOL derivatives bearing multiple stereogenic elements and featuring up to four stereogenic centers. The sequence begins with an organocatalyzed regio- and enantioselective mono-dihydrobenzofurannulation of commercially available 2,7-dihydroxynaphthalene establishing two stereogenic carbon atoms, followed by highly atroposelective copper-catalyzed aerobic oxidative homo- or cross-couplings fixing the axial chirality thanks to a nearly perfect induction of chirality. In addition, the resulting BINOL derivatives serve as promising precursors for the synthesis of either complex spiroheterocycles by axial-to-central conversion of chirality, heterohelicene-like molecules by axial-to-helical conversion of chirality, or an atropisomeric naphtho[2,1-b]furan scaffold via 1,2-aryl migration, further underscoring the potential of this new atroposelective strategy based on a practical central-to-axial chirality induction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


