慢性不可预测应激模型中通过TAAR1受体调节的creb依赖性神经行为改变。
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
CUS扰乱脑细胞和分子生理,导致神经递质和神经内分泌失调,导致神经行为改变,如焦虑、抑郁和认知障碍。许多调节神经递质系统的神经生物学通路与情绪调节和应激反应有关,特别是TAAR1受体已被证明在神经精神疾病中发挥重要作用。本研究在慢性不可预测应激模型中通过TAAR1受体RO5256390调节creb依赖性神经行为改变。为了评估CUS和药物干预对神经行为改变的影响,对瑞士白化小鼠进行了8 周的各种应激刺激。采用EPM、SPT、TST和MWM评估焦虑、抑郁和记忆障碍的行为特征。皮质酮多巴胺,血清素;氧化应激生化参数、炎症介质;同时观察海马和皮层乙酰胆碱酯酶(AChE)活性及组织学变化。给药RO5256390(0.5、1和2 mg/kg i.p),可能通过调节TAAR1的活性,改善了cu暴露小鼠的行为和生化参数。采用氟西汀(20 mg/kg, p.o)作为CUS诱导小鼠的标准参比药物,方便临床比较。研究还发现,经CREB抑制剂666-15(10 mg/kg, i.p.)预处理后,RO5256390的神经保护作用明显减弱,这表明CREB信号通路参与了RO5256390的保护机制减弱。因此,本研究强调了TAAR1-CREB通路在神经保护中的关键作用,以及RO5256390在缓解CUS相关神经行为改变方面的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
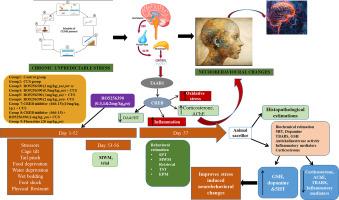
CREB-dependent neurobehavioral alterations via TAAR1 receptor modulation in a chronic unpredictable stress model
CUS disrupts brain cellular and molecular physiology, leading to neurotransmitter and neuroendocrine dysregulation, resulting in neurobehavioral changes like anxiety, depression, and cognitive impairment. Numerous neurobiological pathways regulating neurotransmitter systems, are implicated in mood regulation and stress response, notably the TAAR1 receptor have been documented to play a major role in neuropshychiatric diseases. This research investigates CREB-dependent neurobehavioral alterations via TAAR1 receptor modulation with RO5256390 in Chronic Unpredictable Stress Model. For evaluating the impact of CUS and pharmacological interventions on neurobehavioral changes, Swiss albino mice of either sex were subjected to various stressors for 8 weeks. The behavioral traits for anxiety, depression and memory impairment were assessed by EPM, SPT, TST and MWM. The level of corticosterone dopamine, serotonin; biochemical parameters of oxidative stress, inflammatory mediators; acetylcholinesterase (AChE) activity and histological changes in hippocampus and cortex were also examined. Administration of RO5256390 (0.5, 1 and 2 mg/kg i.p), improved behavioral and biochemical parameters in CUS-exposed mice, most likely, by modulating the activity of TAAR1. Fluoxetine (20 mg/kg, p.o) was employed as a standard reference drug in CUS induced mice to facilitate clinical comparison. It was also observed that neuroprotective effects of RO5256390 were significantly abolished by pre-treatment with 666–15 (10 mg/kg, i.p.), a CREB inhibitor, which signifies the involvement of CREB signaling in attenuating the protective mechanism of RO5256390. Therefore, this study highlights the critical role of TAAR1-CREB pathway in neuroprotection and therapeutic potential of RO5256390 in alleviating neurobehavioral changes associated with CUS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


