多组学分析揭示肉苁茸抗酒精性肝病的机制,通过胆盐水解酶介导SCD1。
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
民族药理学相关性:肉苁蓉,在中医中被尊为“沙漠人参”,历史上被处方治疗五株七伤,滋补五脏。虽然其总糖苷(GCs)显示出对急性酒精性肝病(ALD)的肝保护潜力,但其对慢性ALD的功效及其机制仍未探索。研究目的:本研究旨在通过多组学方法探讨gc对慢性ALD的保护作用,并阐明其分子机制。材料与方法:建立慢性ALD小鼠模型,通过系统评估肝指数、肝功能、氧化应激等肝脏病理特征,评价GCs的治疗效果。综合多组学策略(血浆靶向代谢组学、胆汁酸特异性分析、肝脏转录组学)被用于描述代谢重编程和识别关键信号通路。机制研究表明,GCs降低了胆汁盐水解酶(BSH)的活性。BSH抑制剂咖啡酸苯乙酯(CAPE)被用作GCs机制研究的药理学工具。通过qRT-PCR、Western blot和小干扰RNA (siRNA)介导的基因沉默方法验证了其分子机制。结果:GCs治疗明显减轻ALD病理。血浆靶向代谢组学和胆汁酸谱显示了gc介导的胆汁酸稳态和脂质代谢网络的重塑。从机制上讲,GCs能有效抑制BSH活性,这在ALD的发病机制中由CAPE证实了其关键作用。转录组学和分子分析显示,GCs随后降低SCD1,激活AMPK/mTOR信号,从而协调调节脂质分解代谢和炎症级联反应。结论:这些发现强调了gc对慢性ALD的保护作用,通过抑制BSH活性来控制胆汁酸代谢,从而减轻胆汁淤积,从而调节SCD1/AMPK/mTOR信号通路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
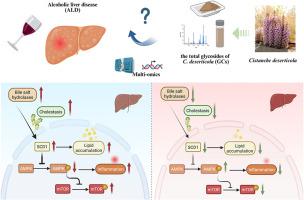
Multi-omics analysis reveals the mechanism of Cistanche deserticola against alcoholic liver disease via bile salt hydrolase and SCD1
Ethnopharmacological relevance
Cistanche deserticola Y. C. Ma, revered as “desert ginseng” in traditional Chinese medicine, has been historically prescribed for treating five strains and seven injuries, and nourishing the five zang organs. While the total glycosides from C. deserticola (GCs) demonstrate hepatoprotective potential against acute alcoholic liver disease (ALD), their efficacy in chronic ALD and mechanism remain unexplored.
Aim of the study
This study aimed to investigate the protective effects of GCs against chronic ALD and to elucidate its molecular mechanisms by multi-omics approach.
Materials and methods
A chronic ALD mouse model evaluated the therapeutic effects of GCs, with systematic assessment of hepatic pathological features including liver index, liver functions, and oxidative stress, etc. Integrated multi-omics strategies (plasma-targeted metabolomics, bile acid-specific profiling, hepatic transcriptomics) were employed to delineate metabolic reprogramming and identify critical signaling pathways. The BSH inhibitor caffeic acid phenethyl ester (CAPE) was used as a pharmacological tool for the mechanistic investigation of GCs. The molecular mechanism was validated by qRT-PCR, Western blot, and small interfering RNA (siRNA)-mediated gene silencing methods.
Results
GCs treatment significantly attenuated ALD pathologies. Plasma-targeted metabolomics and bile acid profiling demonstrated GCs-mediated remodeling of bile acid homeostasis and lipid metabolic networks. Mechanistically, GCs potently inhibited BSH activity, which was validated the pivotal role in ALD pathogenesis by CAPE. Transcriptomic and molecular analyses revealed that GCs subsequently reduced SCD1, and activated AMPK/mTOR signaling, thereby coordinately regulating lipid catabolism, and inflammatory cascades.
Conclusion
These findings highlight the protective effects of GCs against chronic ALD through inhibition of BSH activity to dictate bile acid metabolism, thereby alleviating cholestasis, which subsequently regulate SCD1/AMPK/mTOR signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


