青光安二汤通过抑制TRPV4/NF-κB信号通路减轻青光眼视网膜神经节细胞焦亡。
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
民族药理学相关性:青光眼视神经病变(GON)以视网膜神经节细胞(RGCs)进行性变性为特征,是青光眼不可逆性视力损害的主要病理基础。青光安II汤是一种具有数十年临床应用经验的中药方剂,具有促进视神经修复和保护视野的作用。然而,QGAII神经保护作用的确切机制尚不完全清楚。研究目的:本研究旨在探讨QGAII通过靶向调节TRPV4/NF-κB信号通路减轻RGC焦亡的具体分子机制。材料和方法:采用DBA/2J小鼠慢性高眼压模型和新型青光眼损伤细胞模型,在体内和体外对QGAII的神经保护作用进行了评价。在体内,通过苏木精和伊红(H&E)染色和透射电镜(TEM)评估QGAII干预的效果。通过免疫荧光、流式细胞术、western blot (WB)、qPCR和ELISA进一步研究其作用机制。在体外,采用连续静水加压(CHP)和氧葡萄糖剥夺(OGD)相结合的方法建立新型青光眼细胞损伤模型。采用脂质体转染法探讨TRPV4、NF-κB和GSDMD的作用。随后,将QGAII给予该损伤模型,并通过细胞计数试剂盒-8 (CCK-8)、流式细胞术、扫描电镜(SEM)、免疫荧光、WB、qPCR和ELISA分析其作用,以阐明其通过TRPV4/NF-κB信号通路减弱焦亡的作用。结果:体内H&E染色和TEM显示,QGAII对DBA/2J小鼠视网膜RGC有保护作用,其作用与RGC焦亡减少有关。进一步分析表明,QGAII可能通过TRPV4/NF-κB信号通路减轻了DBA/2J小鼠的RGC焦亡。在体外,我们首先建立了CHP和OGD诱导的细胞损伤模型,表现出gsdmd依赖的焦亡表型。这一过程被证实受TRPV4/NF-κB信号通路调控。随后的QGAII干预降低了RGC焦亡的发生率。免疫荧光、WB、qPCR和ELISA证实,这种保护作用是通过抑制TRPV4/NF-κB信号通路介导的。结论:QGAII通过靶向调节TRPV4/NF-κB信号通路抑制RGC焦亡,为GON的治疗性管理提供有效的视神经保护。本文章由计算机程序翻译,如有差异,请以英文原文为准。
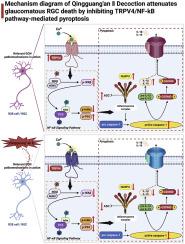
Qingguang’An II decoction attenuates retinal ganglion cell pyroptosis in glaucoma via inhibition of the TRPV4/NF-κB signaling pathway
Ethnopharmacological relevance
Glaucomatous optic neuropathy (GON), characterized by the progressive degeneration of retinal ganglion cells (RGCs), is the primary pathological basis of irreversible visual impairment in glaucoma. Qingguang’An II decoction (QGAII), a traditional Chinese medicine formula with decades of clinical application, has been shown to promote optic nerve repair and preserve the visual field in patients with GON. Nevertheless, the precise mechanisms underlying the neuroprotective effects of QGAII remain incompletely understood.
Aim of the study
This study aimed to investigate the specific molecular mechanisms by which QGAII mitigates RGC pyroptosis through the targeted modulation of the TRPV4/NF-κB signaling pathway.
Materials and methods
The neuroprotective effects of QGAII were evaluated both in vivo using a DBA/2J mouse model of chronic ocular hypertension and in vitro using a novel cell model of glaucomatous injury. In vivo, the effect of QGAII intervention was assessed by hematoxylin and eosin (H&E) staining and transmission electron microscopy (TEM). The mechanism of action was further investigated by immunofluorescence, flow cytometry, Western blot (WB), qPCR, and ELISA. In vitro, a novel glaucomatous cell injury model was established by combining continuous hydrostatic pressure (CHP) and oxygen-glucose deprivation (OGD). Liposome transfection was employed to explore the roles of TRPV4, NF-κB, and GSDMD. Subsequently, QGAII was administered to this injury model, and its effects were analyzed using a cell counting kit-8 (CCK-8), flow cytometry, scanning electron microscopy (SEM), immunofluorescence, WB, qPCR, and ELISA to clarify its role in attenuating pyroptosis via the TRPV4/NF-κB signaling pathway.
Results
In vivo, H&E staining and TEM showed that QGAII exerted a protective effect on RGCs in the retina of DBA/2J mice, and this effect was linked to a reduction in RGC pyroptosis. Further analysis showed that QGAII attenuated RGC pyroptosis in DBA/2J mice, potentially through the TRPV4/NF-κB signaling pathway. In vitro, we first established that the cell injury model, induced by CHP and OGD, exhibited a GSDMD-dependent pyroptotic phenotype. This process was confirmed to be regulated by the TRPV4/NF-κB signaling pathway. Subsequent QGAII intervention reduced the incidence of RGC pyroptosis. This protective effect was mediated by the inhibition of the TRPV4/NF-κB signaling pathway, as confirmed by immunofluorescence, WB, qPCR, and ELISA.
Conclusion
QGAII suppresses RGC pyroptosis through the targeted modulation of the TRPV4/NF-κB signaling pathway, providing effective optic neuroprotection for the therapeutic management of GON.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


