抑制tirap介导的炎症信号:一种有希望的治疗败血症的策略。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
及时治疗败血症中失调的免疫反应对于恢复免疫稳态和防止器官功能障碍的进展至关重要。在这项研究中,我们研究了具有抗炎特性的抗生素和抗炎化合物dorzolamide的联合治疗是否会改善细菌性败血症的预后。使用计算机方法,我们筛选了一个fda批准的抗生素结构库,以确定那些可以与Toll/白细胞介素-1受体(TIR)结构域的连接蛋白(TIRAP)相互作用的抗生素,TIRAP是一种在免疫细胞中调节促炎细胞因子产生的蛋白质。我们的虚拟筛选确定了一种广谱抗生素,左氧氟沙星,作为候选药物。随后,我们采用盲肠浆液(CS)脓毒症小鼠模型在体内验证候选小鼠,同时监测小鼠的存活率、组织mRNA表达、细胞形态、细胞因子水平和其他生化指标。体内研究证实,左氧氟沙星联合多唑胺(LeDoz)可提高脓毒症小鼠的存活率。苏木精和伊红(H&E)组织染色、细胞因子水平、免疫荧光双染色和血清生物标志物均显示,与未治疗的脓毒症小鼠相比,ledoz治疗组的脓毒症小鼠重建了稳态状态。体外分析也证实了LeDoz能够减弱tirap介导的炎症信号。因此,左氧氟沙星和dorzolamide联合使用既具有抗菌作用,又具有通过抑制TIRAP激活协同减少宿主慢性炎症的强大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
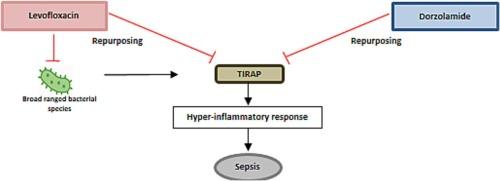
Inhibiting TIRAP-mediated inflammatory signaling: A promising therapeutic strategy against sepsis
The timely therapeutic targeting of the dysregulated immune response in sepsis is essential to restore immune homeostasis and prevent progression to organ dysfunction. In this study, we investigated whether a combination therapy with an antibiotic exhibiting anti-inflammatory properties and the anti-inflammatory compound dorzolamide will improve the bacterial sepsis outcome. Using an in-silico approach, we screened a structure library of FDA-approved antibiotics to identify those that can interact with the Toll/interleukin-1 Receptor (TIR) domain-containing adaptor protein (TIRAP), a protein regulating proinflammatory cytokine production in immune cells. Our virtual screening identified a broad-spectrum antibiotic, levofloxacin, as a candidate. We subsequently employed the cecum slurry (CS) septic mouse model to validate the candidates in vivo, while monitoring survival of mice, tissue mRNA expression, cell morphology, cytokine levels, and other biochemical markers. The in vivo studies confirmed that the combination of levofloxacin and dorzolamide (LeDoz) increased the survival rate of septic mice. Hematoxylin and eosin (H&E) tissue staining, cytokine levels, as well as immunofluorescence dual staining and serum biomarkers, all showed the reestablishment of homeostatic conditions in the LeDoz-treated group of septic mice compared to untreated septic mice. In vitro analyses also confirmed the ability of LeDoz to attenuate TIRAP-mediated inflammatory signaling. Thus, the combination of levofloxacin and dorzolamide exhibits both an antibacterial effect and a strong potential for synergistically reducing chronic inflammation in the host by inhibiting the activation of TIRAP.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


