妊娠 X 受体是登革热病毒感染的一个新的宿主靶点,它重编程脂质代谢并抑制免疫反应。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
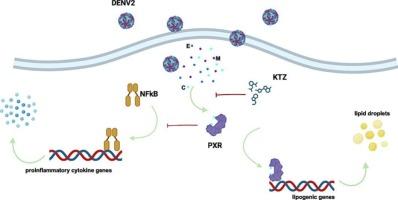
登革热病毒(DENV)是世界上主要的蚊子传播病毒,每年约有50万人发生严重的DENV感染(登革出血热或登革休克综合征)。DENV主要局限于热带和亚热带地区,但由于气候变化和城市化,其范围正在向南北扩大。目前治疗选择有限,迫切需要确定新的抗病毒靶点。在这里,我们证明,在巨噬细胞中,DENV2增加脂滴的生物发生以及参与甘油三酯和胆固醇合成和积累的基因的表达,同时通过激活孕烷 X 受体(PXR)(一种核配体依赖的转录因子)降低免疫反应。冈代酸或酮康唑(KTZ)抑制PXR部分恢复白细胞介素(IL)-1β、IL-6、肿瘤坏死因子-α、IL-12、干扰素(IFN)-γ和IFN-α水平,并阻断DENV2感染诱导的Srebp2、Pparγ、Cd36和Sqle的表达。KTZ是一种PXR拮抗剂和美国食品和药物管理局批准的抗真菌药物,在体外和体内也可以减少病毒的复制。我们还确定了DENV2衣壳蛋白驱动PXR激活。因此,本研究表征了pxr依赖性脂肪酸和胆固醇代谢重编程,以及DENV2促进的免疫抑制,以促进其复制。综上所述,我们的数据将PXR定位为登革热治疗的一个新的可药物靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The pregnane X receptor is a novel host target for dengue virus infection that reprograms lipid metabolism and suppresses the immune response
Dengue virus (DENV) is the main mosquito-transmitted virus in the world, with approximately 500,000 people developing severe DENV infection (dengue hemorrhagic fever or dengue shock syndrome) annually. DENV is localized principally to tropical and subtropical regions, but its range is expanding north and south due to climate change and urbanization. Therapeutic options are currently limited, and the identification of new antiviral targets is urgently needed. Here, we demonstrated that, in macrophages, DENV2 increases lipid droplet biogenesis and the expression of genes involved in triglyceride and cholesterol synthesis and accumulation while decreasing the immune response through the activation of the pregnane X receptor (PXR), a nuclear ligand–dependent transcription factor. PXR inhibition with okadaic acid or ketoconazole (KTZ) partially restored interleukin (IL)-1β, IL-6, tumor necrosis factor-α, IL-12, interferon (IFN)-γ, and IFN-α levels and blocked the DENV2 infection–induced expression of Srebp2, Pparγ, Cd36, and Sqle. KTZ, a PXR antagonist and United States Food and Drug Administration–approved antimycotic drug, also reduced viral replication in vitro and in vivo. We additionally determined that the DENV2 capsid protein drives PXR activation. Thus, the present investigation led to the characterization of the PXR-dependent reprogramming of fatty-acid and cholesterol metabolism and immune suppression promoted by DENV2 to facilitate its replication. Taken together, our data position PXR as a new druggable target for dengue treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


