层状双层氢氧化铝镁吸附磷酸离子的动力学研究
IF 0.6
4区 工程技术
Q4 ENGINEERING, CHEMICAL
引用次数: 0
摘要
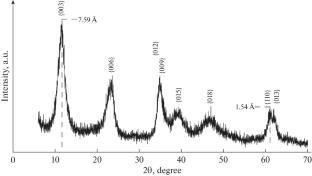
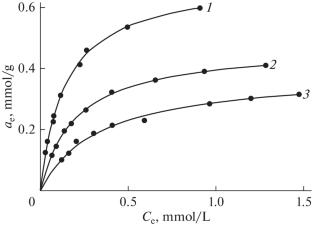
采用固相相互作用法研究了Mg4Al2(OH)12CO3⋅3H2O层状双氢氧化物对\({\text{PO}}_{4}^{{3 - }}\)离子的吸附性能。发现吸附过程用Langmuir单分子吸附方程最能描述,其动力学用拟一阶方程描述。测定了该过程的活化能,据此得出该过程发生在扩散区。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of Phosphate Ion Sorption by Layered Double Magnesium Aluminum Hydroxide
The paper presents the results of studies of the sorption properties of layered double hydroxide of the composition Mg4Al2(OH)12CO3⋅3H2O, obtained by the solid-phase interaction method, with respect to the \({\text{PO}}_{4}^{{3 - }}\) ion. It is found that the sorption process is most adequately described by the Langmuir monomolecular adsorption equation, and its kinetics is described by a pseudo-first-order equation. The activation energy of the process is determined, based on which is concluded that the process occurs in the diffusion region.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
25.00%
发文量
70
审稿时长
24 months
期刊介绍:
Theoretical Foundations of Chemical Engineering is a comprehensive journal covering all aspects of theoretical and applied research in chemical engineering, including transport phenomena; surface phenomena; processes of mixture separation; theory and methods of chemical reactor design; combined processes and multifunctional reactors; hydromechanic, thermal, diffusion, and chemical processes and apparatus, membrane processes and reactors; biotechnology; dispersed systems; nanotechnologies; process intensification; information modeling and analysis; energy- and resource-saving processes; environmentally clean processes and technologies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


