甲基氯硅烷热解分解的第一步
IF 0.6
4区 工程技术
Q4 ENGINEERING, CHEMICAL
引用次数: 0
摘要
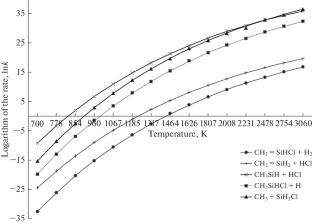
本文对甲基氯硅烷分子热解分解第一步发生的化学反应进行了理论研究。确定了甲基氯硅烷最可能的分解反应及其机理,对不同温度下的产物和过渡态结构进行了热力学计算,并计算了反应的平衡常数和速率常数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The First Step of the Pyrolytic Decomposition of Methylchlorosilane
The article is devoted to the theoretical study of chemical reactions occurring in the first step of the pyrolytic decomposition of the methylchlorosilane molecule. The most probable decomposition reactions of methylchlorosilane and their mechanisms were identified, a thermodynamic calculation of the products and transition state structures at different temperatures was performed, and the equilibrium and rate constants of the reactions were calculated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
25.00%
发文量
70
审稿时长
24 months
期刊介绍:
Theoretical Foundations of Chemical Engineering is a comprehensive journal covering all aspects of theoretical and applied research in chemical engineering, including transport phenomena; surface phenomena; processes of mixture separation; theory and methods of chemical reactor design; combined processes and multifunctional reactors; hydromechanic, thermal, diffusion, and chemical processes and apparatus, membrane processes and reactors; biotechnology; dispersed systems; nanotechnologies; process intensification; information modeling and analysis; energy- and resource-saving processes; environmentally clean processes and technologies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


