各种芳基噻唑氨基喹唑啉衍生物抗癌剂的设计、合成及抗癌评价
IF 2.7
Q2 Chemistry
引用次数: 0
摘要
用1HNMR、13CNMR和质谱对一系列新的芳基噻唑胺类喹啉(10a-j)衍生物的结构进行了表征。此外,所有新开发的(10a-j)衍生物对MCF-7(人乳腺癌)、A549(人肺癌)、Colo-205(人结肠癌)和A2780(人卵巢癌)等4种人类癌细胞系的初步抗癌活性采用MTT法,以依托oposide (etoposide)为阳性对照。与依托泊苷(etoposide)相比,筛选的大多数化合物显示出良好至中等的活性。IC50值范围为0.02±0.0072µM ~ 7.90±2.14µM,阳性对照IC50值范围为0.17±0.034µM ~ 3.34±0.152µM。其中化合物10a、10g、10h、10i和10j表现出较强的活性。其中一个化合物10j的抗肿瘤活性明显优于阳性对照。本文章由计算机程序翻译,如有差异,请以英文原文为准。
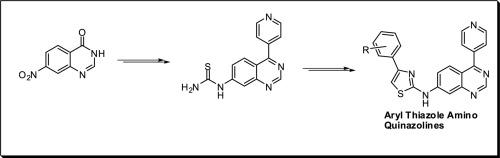
Design, synthesis and anticancer evaluation of various aryl thiazole amino quinazoline derivatives as anticancer agents
A new series of various aryl thiazole amine incorporated quinazoline (10a-j) derivatives and their structures are characterized by 1HNMR, 13CNMR and mass spectral data. Further, all the newly developed (10a-j) derivatives are assessed for their preliminary anticancer activity against four human cancer cell lines, such as MCF-7(human breast cancer), A549 (human lung cancer), Colo-205 (human colon cancer) & A2780 (human ovarian cancer) by employing the MTT method and etoposide (Etoposide) used as a positive control. Most of the screened compounds were displayed good to moderate activity as compared with etoposide (Etoposide). The IC50 values range from 0.02±0.0072 µM to 7.90±2.14 µM, and the positive control showed values ranging from 0.17 ± 0.034 µM to 3.34 ± 0.152 µM respectively. Among the tested derivatives, five compounds 10a, 10g, 10h, 10i and 10j exhibited more potent activity. Mainly, one compound 10j displayed superior anticancer activity than the positive control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Data Collections
Chemistry-Chemistry (all)
CiteScore
6.10
自引率
0.00%
发文量
169
审稿时长
24 days
期刊介绍:
Chemical Data Collections (CDC) provides a publication outlet for the increasing need to make research material and data easy to share and re-use. Publication of research data with CDC will allow scientists to: -Make their data easy to find and access -Benefit from the fast publication process -Contribute to proper data citation and attribution -Publish their intermediate and null/negative results -Receive recognition for the work that does not fit traditional article format. The research data will be published as ''data articles'' that support fast and easy submission and quick peer-review processes. Data articles introduced by CDC are short self-contained publications about research materials and data. They must provide the scientific context of the described work and contain the following elements: a title, list of authors (plus affiliations), abstract, keywords, graphical abstract, metadata table, main text and at least three references. The journal welcomes submissions focusing on (but not limited to) the following categories of research output: spectral data, syntheses, crystallographic data, computational simulations, molecular dynamics and models, physicochemical data, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


