单细胞动力学揭示了应激诱导的新生大鼠心肌细胞肥大和凋亡之间的决定
IF 2.2
Journal of molecular and cellular cardiology plus
Pub Date : 2025-09-16
DOI:10.1016/j.jmccpl.2025.100484
引用次数: 0
摘要
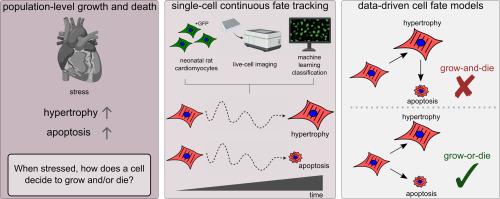
心肌细胞肥大和凋亡是心肌病和心力衰竭的基础。虽然先前的研究已经报道了在群体水平上的肥大和凋亡,但个体细胞如何承担这些不同的模拟和数字命运尚不清楚。为了阐明单个细胞如何决定生长和/或死亡,我们开发了一种高含量显微镜方法来跟踪新生大鼠心肌细胞的单细胞动力学。即使未经处理的细胞在生长和死亡方面也表现出明显的单细胞变异性。staurosporine或phenylephrine的统一处理分别诱导了不同的形态学程序,导致细胞凋亡和肥大,但仅在细胞亚群中。增加β-肾上腺素能受体激动剂异丙肾上腺素的浓度可引起群体水平的肥厚和细胞凋亡双相诱导,这与大多数肥厚细胞的凋亡(生长-死亡模型)或肥厚和延迟细胞凋亡之间的早期决定(生长-死亡模型)相一致。通过追踪单细胞的命运,我们发现,在异丙肾上腺素或苯肾上腺素的胁迫下,肥大的单个细胞受到保护,免受后来的凋亡。此外,caspase 3的抑制使单细胞的可能性从凋亡转变为肥大。机器学习模型发现,细胞的初始大小和DNA含量或凝结可以预测细胞的肥大或凋亡倾向。总之,这些数据支持心肌细胞决策的生长或死亡概念模型。这种用于跟踪模拟-数字联合细胞决策的单细胞分析方法表明,尽管肥大和凋亡在群体水平上共同发生,但单个心肌细胞很早就决定了是生长还是死亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Single-cell dynamics reveal a stress-induced decision between hypertrophy and apoptosis in neonatal rat cardiomyocytes
Cardiomyocyte hypertrophy and apoptosis underlie cardiomyopathies and heart failure. While previous studies have reported both hypertrophy and apoptosis at the population level, how individual cells commit to these distinct analog and digital fates is unclear. To elucidate how individual cells decide to grow and/or die, we developed a high-content microscopy approach to track single-cell dynamics of neonatal rat cardiomyocytes. Even untreated cells exhibited substantial single-cell variability in growth and death. Uniform treatments of staurosporine or phenylephrine induced distinctive morphological programs resulting in apoptosis and hypertrophy, respectively, but only in cell subpopulations. Increasing concentrations of the β-adrenergic receptor agonist isoproterenol caused a population-level biphasic induction of hypertrophy and then apoptosis, consistent with either apoptosis in the most hypertrophic cells (a grow-and-die model) or an early decision between hypertrophy and delayed apoptosis (a grow-or-die model). By tracking single-cell fates, we found that when stressed with either isoproterenol or phenylephrine, individual cells that hypertrophy are protected from later apoptosis. Further, caspase 3 inhibition shifted the single-cell probability from apoptosis to hypertrophy fates. Machine learning models found that a cell's initial size and DNA content or condensation could predict a cell's bias for hypertrophy or apoptosis. Together, these data support a grow-or-die conceptual model for cardiomyocyte decisions. This single-cell profiling method for tracking joint analog-digital cell decisions reveals that despite hypertrophy and apoptosis co-occurring at the population level, individual cardiomyocytes decide early whether to grow or die.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular and cellular cardiology plus
Cardiology and Cardiovascular Medicine
自引率
0.00%
发文量
0
审稿时长
31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


