羟肟酸衍生物通过诱导氧化应激和DNA损伤作为有效的抗胃癌药物的发现
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胃癌(Gastric cancer, GC)是世界上发病率和死亡率最高的恶性肿瘤之一,迫切需要开发新的胃癌治疗药物。在此,我们评估了一系列羟酸衍生物3a-3k对GC细胞的抗增殖活性,发现化合物3i可以在微摩尔水平上抑制GC细胞的增殖。随后的细胞生长曲线、细胞形态、细胞周期和细胞凋亡实验表明,化合物3i通过诱导细胞凋亡而不是抑制细胞周期来抑制GC细胞的生长。此外,拯救实验表明,抗氧化剂(n -乙酰半胱氨酸和二硫苏糖醇)代替细胞凋亡、坏死或铁凋亡抑制剂能完全拯救化合物3i阻止的GC细胞的增殖。随后,RNA-seq、western blotting和碱性彗星试验研究表明,化合物3i可引起DNA损伤。体内实验结果一致表明,化合物3i能明显抑制肿瘤的生长,诱导p53和γ - h2ax的表达。综上所述,化合物3i的抗增殖活性是通过增加ROS水平,诱导DNA损伤,从而导致GC细胞凋亡。此外,对接研究表明化合物3i能够螯合位于hdac活性位点的Zn2+。因此,化合物3i为开发通过氧化应激治疗GC的先导化合物提供了重要的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
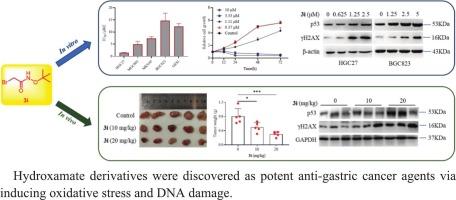
Discovery of hydroxamate derivatives as potent anti-gastric cancer agents via inducing oxidative stress and DNA damage
Gastric cancer (GC) remains one of the most malignant cancers with high morbidity and mortality, necessitating the development of new agent for GC treatment. Herein, we evaluated the anti-proliferative activities of a series of hydroxamate derivatives 3a-3k against GC cells, and found that compound 3i could inhibit the proliferation of GC cells at micromolar level. The subsequent cell growth curve, cell morphology, cell cycle and cell apoptosis experiments indicated that compound 3i inhibited the growth of GC cells via inducing cell apoptosis rather than suppressing cell cycle. Besides, the rescue experiments showed that antioxidants (N-acetylcysteine and dithiothreitol) instead of cell apoptosis, necrosis or ferroptosis inhibitors could totally rescue the proliferation of GC cells prevented by compound 3i. Then, RNA-seq, western blotting and alkaline comet assay studies showed that compound 3i could cause DNA damage. Consistently, the in vivo experiments indicated that compound 3i could obviously inhibit the growth of tumor and induce the expression of p53 and γH2AX. Overall, compound 3i exhibited its anti-proliferative activity through increasing ROS level and inducing DNA damage, thus resulting in cell apoptosis of GC cells. Furthermore, docking study exposed the ability of compound 3i to chelate Zn2+ located within HDACs active site. Therefore, compound 3i provides an important strategy on developing lead compounds for GC therapy via oxidative stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


