抑制可溶性环氧化物水解酶改善高同型半胱氨酸血症诱导的血管周围脂肪组织功能障碍:揭示环氧二碳三烯酸作为血管周围脂肪组织来源的松弛因子
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
目的高同型半胱氨酸血症损害血管内皮和平滑肌。我们最近报道了同型半胱氨酸也会损害血管周围脂肪组织(PVAT)功能,但其机制尚不清楚。本研究旨在通过揭示环氧二碳三烯酸(EETs)在PVAT抗收缩/血管舒张活性中的作用,进一步了解高同型半胱氨酸血症诱导的PVAT功能障碍的机制。进一步探讨了靶向可溶性环氧化物水解酶(sEH)对高同型半胱氨酸血症诱导的PVAT功能障碍的作用。方法采用蛋氨酸饮食诱导高同型半胱氨酸血症大鼠模型和体外同型半胱氨酸培养模型。研究了PVAT和内皮完整(PVAT + E+)或同时剥离(PVAT-E−)、PVAT (PVAT-E+)或内皮剥离(PVAT + E−)主动脉环的血管反应性。测定主动脉PVAT中EETs、sEH的表达和活性,并转移至骨化主动脉进行血管反应性调节研究。结果体内和体外研究表明,同型半胱氨酸增强了血管对苯肾上腺素和KCl的收缩反应,减弱了血管对乙酰胆碱的松弛反应。高同型半胱氨酸血症大鼠(8,9-,11,12-,14,15- eet)和暴露于同型半胱氨酸(11,12-,14,15- eet)后,主动脉PVAT中的eet降低,与sEH的表达和活性增加有关。用sEH抑制剂TPPU处理体内和体外模型可抑制sEH活性,增加PVAT中的eet,同时恢复PVAT的抗收缩/血管舒张能力。tppu处理的高同型半胱氨酸大鼠PVAT抑制了高同型半胱氨酸大鼠主动脉的收缩力。11,12-和14,15- eet均可引起pat - e -主动脉松弛。结论seets是pvat衍生的松弛因子。抑制sEH可防止PVAT中同型半胱氨酸诱导的eet丢失,从而提高PVAT的抗收缩/血管舒张活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
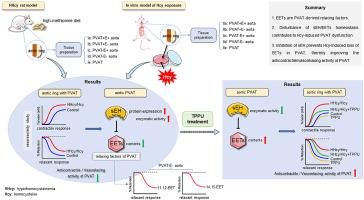
Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced perivascular adipose tissue dysfunction: Revealing epoxyeicosatrienoic acid as a perivascular adipose tissue-derived relaxing factor
Objectives
Hyperhomocysteinemia harms vascular endothelium and smooth muscle. We recently reported that homocysteine also impairs perivascular adipose tissue (PVAT) function but the mechanisms remain poorly elucidated. This study aimed to advance mechanistic understanding of hyperhomocysteinemia-induced PVAT dysfunction through revealing the role of epoxyeicosatrienoic acids (EETs) in the anticontractile/vasorelaxing activity of PVAT. Further, the effect of targeting soluble epoxide hydrolase (sEH) against hyperhomocysteinemia-induced PVAT dysfunction was explored.
Methods
Methionine diet-induced hyperhomocysteinemic rat model and in vitro homocysteine-incubation model were used. PVAT and endothelium-intact (PVAT + E+) or both denuded (PVAT-E−), either PVAT (PVAT-E+) or endothelium-denuded (PVAT + E−) aortic rings were studied for vasoreactivity. Aortic PVAT was measured for EETs, sEH expression and activity, and transferred to skeletonized aorta for vasoreactivity regulation study.
Results
Both in vivo and in vitro studies showed that homocysteine augments vasocontractile responses to phenylephrine and KCl and attenuates vasorelaxant response to acetylcholine in PVAT-intact rat aortas with or without endothelium. EETs in the aortic PVAT were decreased in hyperhomocysteinemic rats (8,9-, 11,12-, 14,15-EET) and after homocysteine-exposure (11,12-, 14,15-EET), associating with an increased expression and activity of sEH. Treating in vivo and in vitro models with sEH inhibitor TPPU suppressed sEH activity and increased EETs in the PVAT, accompanied by a restored anticontractile/vasorelaxing capacity of PVAT. PVAT from TPPU-treated hyperhomocysteinemic rat suppressed the contractility of hyperhomocysteinemic rat aorta. Both 11,12- and 14,15-EET evoked relaxation in PVAT-E− aorta.
Conclusions
EETs are PVAT-derived relaxing factors. Inhibition of sEH prevents homocysteine-induced EETs loss in PVAT, thereby improving the anticontractile/vasorelaxing activity of PVAT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


