带电荷的共肽在二氧化硅上的吸附和自组装
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究探讨了驱动肽-二氧化硅相互作用的分子机制,重点研究了赖氨酸-亮氨酸共肽的吸附和自组装及其对溶菌酶吸附的影响。利用经典的长时间尺度分子动力学(MD)模拟,我们研究了电荷密度和序列块性如何影响带负电荷的二氧化硅表面上的肽行为。聚赖氨酸均多肽表现出强烈的静电相互作用,导致表面完全吸附,而中性聚亮氨酸多肽则表现出微弱的吸附和垂直定向。固定电荷密度为0.5的共肽表现出依赖于嵌段的吸附和结构组织。增加的块性促进了肽的独立行为和不同的自组装。与裸二氧化硅相比,用这些肽功能化的改性二氧化硅表面增强了溶菌酶的稳定性,减少了构象破坏。我们的研究结果表明,肽序列设计可以调节表面相互作用和蛋白质吸附。这项工作为工程两亲性多肽的表面功能化提供了有价值的见解,在生物材料、生物传感和治疗递送系统中具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
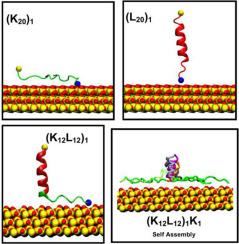
Molecular insights into the adsorption and self-assembly of charged copolypeptides on silica
This study explores the molecular mechanisms driving peptide-silica interactions, focusing on the adsorption and self-assembly of lysine-leucine copolypeptides and their influence on lysozyme adsorption. Using classical, long-timescale molecular dynamics (MD) simulations, we examine how charge density and sequence blockiness affect peptide behavior on negatively charged silica surfaces. Poly-lysine homopolypeptides show strong electrostatic interactions, leading to full surface adsorption, while neutral poly-leucine peptides weakly adsorb and orient perpendicularly. Copolypeptides with a fixed charge density of 0.5 exhibit blockiness-dependent adsorption and structural organization. Increased blockiness promotes independent peptide behavior and varied self-assembly. Modified silica surfaces functionalized with these peptides enhance lysozyme stability and reduce conformational disruption compared to bare silica. Our findings demonstrate that peptide sequence design can modulate surface interactions and protein adsorption. This work provides valuable insights into engineering amphiphilic polypeptides for surface functionalization, with potential applications in biomaterials, biosensing, and therapeutic delivery systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


