影响蛋白质稳定性和相互作用的ATG101-ATG13蛋白复合体界面突变的综合定位:自噬调节的意义
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
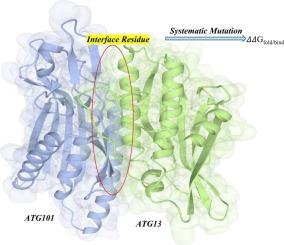
自噬是一种高度协调的细胞过程,对维持细胞稳态至关重要,涉及自噬体的形成,自噬体是一种吞噬和降解细胞成分的双膜结构。这一过程的核心是ATG13和ATG101等蛋白质,它们除了参与ATG9/ATG9A的转运和定位外,还发挥着多方面的作用。ATG13和ATG101共同作为中心支架单元,介导各种自噬起始亚复合物之间的相互作用网络。挑战在于阐明单个蛋白质和复合物的生化和生物物理功能,以及了解它们在自噬体生物发生和自噬执行中的协同作用。在此背景下,我们对ATG101-ATG13复合物内的界面残基进行了硅饱和诱变分析,以预测突变对复合物稳定性和相互作用的影响。在分析了总共1102个蛋白质复合物的界面突变后,我们发现这些变化中的大多数降低了结合亲和力并使蛋白质不稳定。这些结果揭示了突变如何影响自噬成分的稳定性和结合,可以有效地预测突变对蛋白质复合物功能的功能影响。这一信息可能有助于研究人员了解自噬过程的复杂性及其与神经退行性疾病的关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comprehensive mapping of interfacial mutations in the ATG101-ATG13 protein complex that affect protein stability and interaction: Implications in autophagy regulation
Autophagy, a highly orchestrated cellular process crucial for maintaining cellular homeostasis, involves the formation of autophagosomes, double-membraned structures that engulf and degrade cellular components. Central to this process are proteins such as ATG13 and ATG101, which play multifaceted roles beyond their involvement in ATG9/ATG9A trafficking and localization. Together, ATG13 and ATG101 serve as central scaffolding units, mediating a network of interactions among various autophagy initiation subcomplexes. The challenge lies in elucidating the biochemical and biophysical functions of individual proteins and complexes, as well as understanding their collaborative roles in autophagosome biogenesis and autophagy execution. In this context, we conducted an in-silico saturation mutagenesis analysis of interfacial residues within the ATG101-ATG13 complex to predict the impact of mutations on complex stability and interaction. After analyzing a total of 1102 interfacial mutations in protein complexes, we found that the majority of these changes reduced the binding affinity and destabilized the protein. These results provide insight into how mutations affect the stability and binding of autophagic components, which can effectively predict the functional effects of mutations on the function of protein complexes. This information may help researchers understand the complexities of the autophagy process and how it relates to neurodegenerative diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


