利用结构-活性关系将FLAP抑制剂BRP-7重新定位为有效和选择性的sEH抑制剂
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
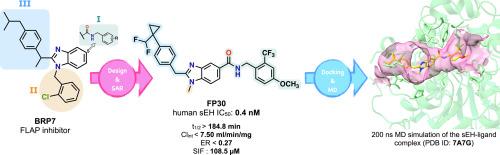
可溶性环氧化物水解酶(sEH)已成为炎症相关疾病,特别是心血管、代谢和中枢神经系统疾病的有效治疗靶点。在这项研究中,我们报道了一类新的基于苯并咪唑的酰胺衍生物的合理设计,合成和生物学评价,这些衍生物是有效的和选择性的sEH抑制剂。这些化合物是通过先前报道的FLAP抑制剂BRP-7的支架优化开发的,通过在苯并咪唑核心的C(2)和C(5)位置进行战略性修饰,以SAR为指导。在合成的类似物中,FP30 (BRP-821)表现出优异的亚纳米级sEH抑制活性(IC50 = 0.4 nM),在人肝微粒体中具有良好的代谢稳定性(t1/2 > 184 min, ER < 0.27),在模拟肠液中具有高溶解度(108 μM)。值得注意的是,先导化合物对FLAP表现出高选择性,将这种新的化学型与双重抑制剂区分开来。总的来说,这些发现突出了一个有希望的新支架,用于进一步优化开发用于神经性疼痛和炎症性疾病治疗的seh靶向疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Harnessing structure-activity relationships to repurpose the FLAP inhibitor BRP-7 into potent and selective sEH inhibitors
Soluble epoxide hydrolase (sEH) has emerged as a validated therapeutic target in inflammation-related conditions, particularly in cardiovascular, metabolic, and central nervous system disorders. In this study, we report the rational design, synthesis, and biological evaluation of a new class of benzimidazole-based amide derivatives as potent and selective inhibitors of sEH. These compounds were developed by scaffold optimization of BRP-7, a previously reported FLAP inhibitor, through strategic modifications at the C(2) and C(5) positions of the benzimidazole core, guided by SAR insights. Among the synthesized analogs, FP30 (BRP-821) exhibited exceptional sub-nM sEH inhibitory activity (IC50 = 0.4 nM), along with excellent metabolic stability in human liver microsomes (t1/2 > 184 min, ER < 0.27) and high solubility in simulated intestinal fluid (108 μM). Notably, the lead compounds demonstrated high selectivity over FLAP, distinguishing this new chemotype from dual inhibitors. Collectively, these findings highlight a promising new scaffold for further optimization toward the development of sEH-targeted therapeutics for the treatment of neuropathic pain and inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


