具有抗sars - cov -2活性的异恶唑嘧啶衍生物的模块化合成
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
建立了一条制备异恶唑嘧啶衍生物的高效合成路线,具有反应条件温和、操作简单、收率高等特点。在此基础上,我们进一步功能化了异恶唑嘧啶支架,合成了7-morpholinoisoxazolo[4,5-d]嘧啶衍生物。体外评价显示,化合物5a和6g具有显著的抗sars - cov -2活性。机制研究表明,5a和6g的抗病毒作用是通过抑制PI3Kδ激酶介导的,突出了它们作为靶向治疗剂的潜力。此外,在金仓鼠模型中进行的体内实验表明,化合物5a有效抑制了SARS-CoV-2在肺组织中的复制,强调了其对抗COVID-19的治疗潜力。这些发现共同表明,异恶唑嘧啶衍生物,特别是5a,代表了作为抗sars - cov -2治疗药物进一步开发的有希望的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
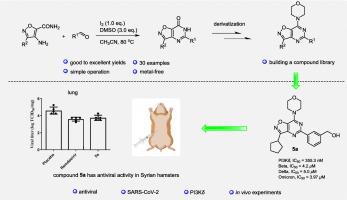
Modular synthesis of isoxazolopyrimidine derivatives with anti-SARS-CoV-2 activity
A highly efficient synthetic route for the preparation of isoxazolopyrimidine derivatives has been developed, characterized by mild reaction conditions, operational simplicity and high yields. Building on this, we further functionalized the isoxazolopyrimidine scaffold to synthesize 7-morpholinoisoxazolo[4,5-d]pyrimidine derivatives. In vitro evaluations revealed that compounds 5a and 6g demonstrated significant anti-SARS-CoV-2 activity. Mechanistic studies indicated that the antiviral effects of 5a and 6g were mediated through the inhibition of PI3Kδ kinase, highlighting their potential as targeted therapeutic agents. Moreover, in vivo experiments conducted in a golden hamster model demonstrated that compound 5a effectively suppressed SARS-CoV-2 replication in lung tissue, underscoring its therapeutic potential for combating COVID-19. These findings collectively suggest that isoxazolopyrimidine derivatives, particularly 5a, represent promising candidates for further development as anti-SARS-CoV-2 therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


