P2X7R-NF-κB-PDI信号通路通过调节atf4介导的xCT上调、ASCT2的s -亚硝基化和小鼠海马GSHS表达,调控lps诱导的GSH合成受损。
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
蛋白二硫异构酶(PDI)通过整合toll样受体4 (TLR4)和P2X7受体(P2X7R)信号通路,以正反馈的方式增强脂多糖(LPS)诱导的核因子-κB (NF-κB)激活。然而,PDI是否参与了P2X7R介导的谷胱甘肽(GSH)生物合成,以响应LPS,这在很大程度上是未知的。在本研究中,lps诱导的NF-κB激活增加了PDI表达,但降低了P2X7+/+小鼠海马中溶质载体1 A5 (ASCT2)水平。PDI敲低可减弱ASCT2下调和s -亚硝基化(SNO-) ASCT2水平对LPS的响应。lps诱导的NF-κB-PDI激活也增加了星形胶质细胞中活化转录因子4 (ATF4)的表达,从而引起胱氨酸:谷氨酸转运蛋白(xCT)的上调,但降低了ASCT2和GSH合成酶(GSHS)的表达。此外,PDI的s -亚硝基化调节了atf4介导的xCT对LPS的上调。SN50(一种NF-κB抑制剂)、PDI下调和ATF4 siRNA均可减轻LPS诱导的GSH含量下降。生理条件下,P2X7R缺失不影响基础PDI、ATF4、xCT和SNO-ASCT2水平。然而,ASCT2表达升高,SNO-PDI水平降低。P2X7R消融改善了(1)PDI、ATF4和xCT2的上调,(2)ASCT2和PDI的s -亚硝基化,(3)ASCT2对LPS的下调。这些发现表明P2X7R-NF-κB-PDI信号通路可能通过调节ASCT2的表达/ s -亚硝基化和atf4介导的xCT调节来抑制LPS对GSH的生物合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
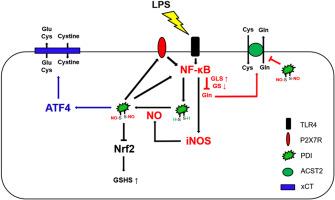
P2X7R-NF-κB-PDI signal pathway regulates LPS-induced impaired GSH synthesis by modulating ATF4-mediated xCT upregulation, S-nitrosylation of ASCT2 and GSHS expressions in the mouse hippocampus
Protein disulfide isomerase (PDI) augments lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) activation by integrating Toll-like receptor 4 (TLR4) and P2X7 receptor (P2X7R) signaling pathways in a positive feedback manner. However, it has been largely unknown whether PDI is involved in altered glutathione (GSH) biosynthesis, which is mediated by P2X7R, in response to LPS. In the present study, LPS-induced NF-κB activation increased PDI expression, but decreased solute carrier 1 A5 (ASCT2) level in the P2X7+/+ mouse hippocampus. PDI knockdown attenuated ASCT2 downregulation and S-nitrosylated (SNO-) ASCT2 level in response to LPS. This LPS-induced NF-κB-PDI activation also increased activating transcription factor 4 (ATF4) expression in astrocytes, which elicited cystine:glutamate transporter (xCT) upregulation, but decreased ASCT2 and GSH synthetase (GSHS) expression. Furthermore, S-nitrosylation of PDI modulated ATF4-mediated xCT upregulation in response to LPS. SN50 (a NF-κB inhibitor), PDI knockdown and ATF4 siRNA mitigated the decreased GSH content induced by LPS. Under physiological condition, P2X7R deletion did not affect basal PDI, ATF4, xCT and SNO-ASCT2 levels. However, it increased ASCT2 expression and decreased SNO-PDI level. P2X7R ablation ameliorated (1) PDI, ATF4 and xCT2 upregulations, (2) S-nitrosylation of ASCT2 and PDI and (3) ASCT2 downregulation in response to LPS. These findings indicate that P2X7R-NF-κB-PDI signal pathway may inhibit GSH biosynthesis in response to LPS by modulating expression/S-nitrosylation of ASCT2 and ATF4-mediated xCT regulation in response to LPS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


