利用ANFIS和遗传算法对颈椎生物力学模型进行校准和实验验证的新方法。
IF 6.3
2区 医学
Q1 BIOLOGY
引用次数: 0
摘要
颈椎是人体生理的重要结构,但易患病变、椎间盘突出和椎体骨折等疾病。有限元(FE)模型是预测不同载荷条件下颈椎生物力学的一种有效方法。传统的方法通常不考虑材料特性的一致性,这可能会限制它们真实地表示生物力学行为的能力。本研究提出了一种新的校准方法,旨在提高颈椎生物力学有限元模型的预测生物保真度。提出的方法系统地确定最佳材料特性,以更好地复制体外生物力学反应。为了实现这一目标,通过计算机断层扫描(CT)重建颈椎几何形状,并根据尸体形态测量数据进行验证,以确保解剖精度。在全面查阅文献的基础上,收集了软硬组织的力学性能。通过使用一系列材料特性进行有限元模拟,生成了生物力学响应数据集。校准过程将自适应神经模糊推理系统(ANFIS)框架与遗传算法集成在一起,随后是针对实验基准的严格验证步骤,以确保体外测试结果的精确复制。结果表明,该校正后的有限元模型显著改善了颈椎生物力学预测,准确匹配了椎间盘力学和韧带行为。该研究提供了一个强大的框架,将数值模拟与实验研究相结合,指导未来的生物力学研究,以潜在地改善临床和手术结果。一些突出的应用是损伤分析、退行性疾病建模和脊柱畸形预测。本文章由计算机程序翻译,如有差异,请以英文原文为准。
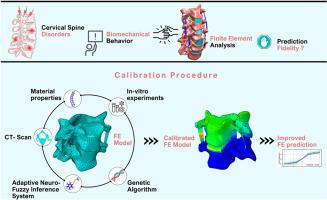
A novel methodology for calibration and experimental validation of cervical spine biomechanics models using ANFIS and genetic algorithm
The cervical spine is an essential structure in human physiology but is vulnerable to diseases, such as lesions, disc herniation, and vertebral fractures. Finite element (FE) modeling represents a powerful approach for predicting the biomechanics of the cervical spine under various loading conditions. Conventional methods usually do not take into account the consistency of the material properties, which potentially restricts their capability to realistically represent biomechanical behavior. This study presents a novel calibration methodology aimed at enhancing the predictive biofidelity of FE models for cervical spine biomechanics. The proposed approach systematically identifies optimal material properties to better replicate in vitro biomechanical responses. To achieve this, the cervical spine geometry was reconstructed from computed tomography (CT) scans and validated against cadaveric morphometric data to ensure anatomical accuracy. Mechanical properties of both hard and soft tissues were collected based on a comprehensive review of the literature. A dataset of biomechanical responses was generated through FE simulations using a range of material properties. The calibration process integrated an adaptive neuro-fuzzy inference system (ANFIS) framework with genetic algorithms, followed by a rigorous validation step against experimental benchmarks to ensure precise replication of in vitro test outcomes. The results show that this calibrated FE model significantly improves cervical spine biomechanics predictions, accurately matching intervertebral disc mechanics and ligament behavior. This research provides a robust framework, integrating numerical modeling with experimental studies, guiding future biomechanical research to potentially improve clinical and surgical outcomes. Some of the prominent applications are injury analysis, degenerative disease modeling, and the prediction of spinal deformity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


