人类胆碱转运体的多样性:底物特异性、动力学性质和抑制剂敏感性。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
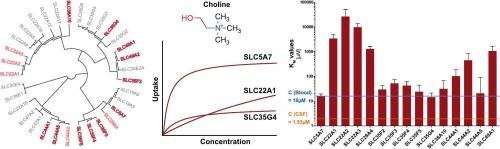
载体介导的胆碱摄取对乙酰胆碱生物合成和各种其他生物过程具有限速作用。迄今为止,已经确定了16种溶质载体(SLC)蛋白,它们可能促进胆碱通过外细胞膜的渗透。然而,它们的生化功能尚未得到实验比较。我们过表达了16种已知胆碱转运能力的SLC蛋白,并比较了它们的胆碱转运动力学。此外,我们评估了它们运输胆碱类似物以及参与其生物合成和降解的代谢物或胆碱能神经传递调节剂的能力,以深入了解slc的生物学功能。此外,我们还研究了转运蛋白是否可以通过它们对钬-3和其他物质抑制的敏感性来区分。在16种slc中,7种(SLC5A7、SLC35F2、SLC35F3、SLC35F4、SLC25F5、SLC35G4和SLC44A5)胆碱转运KM值在12 ~ 50 µM范围内,与生理血浆胆碱浓度密切相关。其中,SLC5A7的固有清除率比其他任何一种都高10倍以上。SLC22A1-3被证实是胆碱转运蛋白,尽管其亲和力较低。3对SLC5A7的抑制作用最强,对SLC35F2-5和SLC35G4的抑制作用也显著。在100 µM的氘-22和维拉帕米溶液中,这6种转运体的胆碱转运被抑制了约50% %。在人类中,多种SLCs可能有助于细胞胆碱摄取,这取决于生理条件和它们尚未完全表征的表达模式。目前的数据还可以增强我们对这些转运蛋白的遗传和环境调节的理解,例如对运动和认知功能的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Diversity of human choline transporters: substrate specificity, kinetic properties, and inhibitor sensitivity
Carrier-mediated uptake of choline is rate-limiting for acetylcholine biosynthesis and various other biological processes. To date, 16 solute carrier (SLC) proteins have been identified that may facilitate choline permeation across the outer cell membrane. However, their biochemical functions have not yet been experimentally compared.
We overexpressed 16 SLC proteins with known choline-transporting capacity and compared their choline transport kinetics. Additionally, we evaluated their capacity to transport choline analogues as well as metabolites involved in its biosynthesis and degradation or modulators of cholinergic neurotransmission to gain insight into the biological functions of the SLCs. Furthermore, we investigated whether the transporters could be distinguished by their sensitivity to inhibition by hemicholinium-3 and other substances.
Of the 16 SLCs tested, seven (SLC5A7, SLC35F2, SLC35F3, SLC35F4, SLC25F5, SLC35G4, and SLC44A5) exhibited KM values for choline transport in the range of 12 to 50 µM, closely aligning with physiological plasma choline concentrations. Among them, SLC5A7 displayed over tenfold higher intrinsic clearance than any of the others. SLC22A1-3 were confirmed as choline transporters, albeit with low affinity. Hemicholinium-3 most strongly inhibited SLC5A7 and also significantly inhibited SLC35F2-5 and SLC35G4. Choline transport by these six transporters was inhibited by about 50 % at 100 µM decynium-22 and verapamil..
In humans, multiple SLCs may contribute to cellular choline uptake, depending on physiologic conditions and their yet incompletely characterized expression patterns. The present data may also enhance our understanding of inherited and environmental modulation of these transporters with possible consequence, for instance, on motor and cognitive functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


