1,3,4-噻二唑衍生物作为炎症和糖尿病双作用酶抑制剂的合成和生物学评价。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
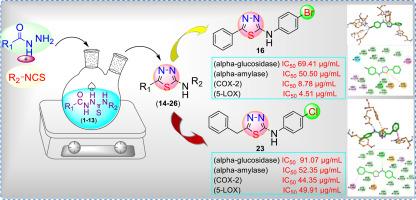
炎症和代谢紊乱(如糖尿病)有共同的复杂途径驱动疾病进展,强调迫切需要多靶点治疗来有效管理。本研究合成了一系列噻唑核心第5位苯基和苄基取代的1,3,4-噻二唑衍生物(14-26),并用FT-IR、1H NMR、13C NMR和EI-MS等光谱技术对其进行了表征。生物学评价显示,与2型糖尿病相关的关键炎症酶(COX-2和5-LOX)和代谢酶(α-葡萄糖苷酶和α-淀粉酶)有良好的抑制作用。化合物16的IC50值分别为8.78 μg/ml (COX-2)、4.51 μg/ml (5-LOX)、69.41 μg/ml (α-葡萄糖苷酶)和50.50 μg/ml (α-淀粉酶),与标准抑制剂相比,具有较强的抑制作用。化合物26的IC50值分别为2.03 μg/ml (COX-2)和6.03 μg/ml (5-LOX)。分子对接研究进一步支持了生物学上的发现,揭示了这些衍生物与目标酶活性位点的结合相互作用,增强了它们作为双作用抗炎和抗糖尿病药物的主要候选药物的潜力。这些发现表明1,3,4-噻二唑衍生物有潜力发展成为针对炎症和代谢失调的多功能治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluations of 1,3,4-thiadiazole derivatives as dual acting enzyme inhibitors to target inflammation and diabetes
Inflammation and metabolic disorders like diabetes mellitus share complex pathways that drive disease progression, emphasizing the urgent need for multi-target therapeutics for effective management. In the present study, a series of 1,3,4-thiadiazole derivatives (14–26) bearing phenyl and benzyl substitutions at fifth position of thiadiazole core were synthesized and characterized with spectroscopic techniques like FT-IR,1H NMR,13C NMR and EI-MS. The biological evaluation revealed promising inhibition of the key inflammatory enzymes (COX-2 and 5-LOX), and metabolic enzymes (α-glucosidase and α-amylase) linked to type 2 diabetes. Among the tested derivatives, compound 16 exhibited the most potent dual action, with IC50 values of 8.78 μg/ml (COX-2), 4.51 μg/ml (5-LOX), 69.41 μg/ml (α-glucosidase), and 50.50 μg/ml (α-amylase), demonstrated moderated activity as compared to the standard inhibitors. Compound 26 also exhibited significant anti-inflammatory, with IC50 values of 2.03 μg/ml (COX-2), 6.03 μg/ml (5-LOX). Molecular docking studies further supported the biological findings, revealing binding interactions of these derivatives with the active sights of targeted enzymes, reinforcing their potential as lead candidates for dual acting anti-inflammatory and anti-diabetic agents. These findings suggest that 1,3,4-thiadiazole derivatives have the potential to develop as multi-functional therapeutics to target inflammation and metabolic dysregulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


