含糖饮料中的果糖和葡萄糖通过SORD促进结直肠癌转移。
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
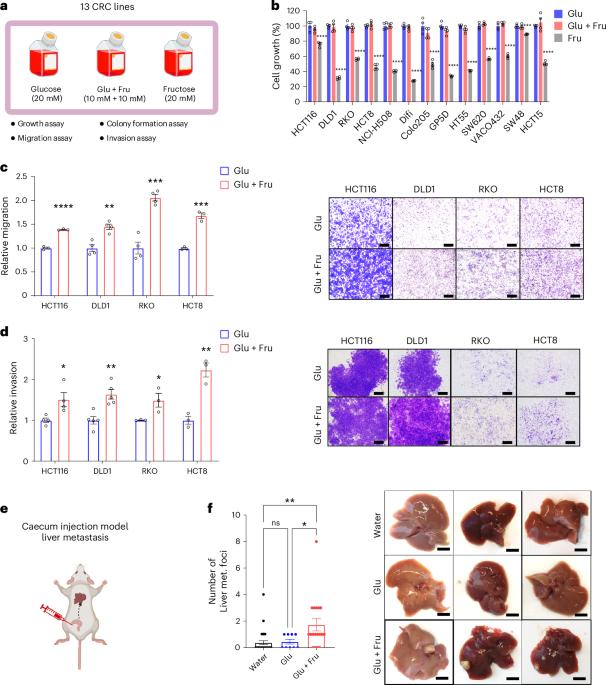
含糖饮料(SSBs)含有高水平的果糖和葡萄糖,与结直肠癌(CRC)风险增加有因果关系和机制联系。然而,食用SSB对晚期疾病进展(包括转移)的影响仍然知之甚少。本研究表明,与单独葡萄糖相比,将结直肠癌细胞暴露于葡萄糖和果糖配方中(反映了ssbs中发现的高果糖玉米糖浆和蔗糖的成分)可增强细胞活力和转移潜力。考虑到CRC细胞在单独的果糖环境下生长不良,并且体内细胞不会在生理上暴露于没有葡萄糖的果糖环境中,除非需要作为对照,否则我们将仅果糖条件从我们的研究中排除。机制上,葡萄糖和果糖的结合通过激活多元醇途径中山梨醇脱氢酶的逆反应,提高了NAD + /NADH的比值。这种氧化还原转变缓解了NAD⁺的局限性,并加速了糖酵解活性,这反过来又激活了甲羟戊酸途径,最终促进了CRC细胞的运动和转移。我们的研究结果强调了SSBs对结直肠癌进展的有害影响,并提出了减轻结直肠癌患者转移的潜在饮食和治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fructose and glucose from sugary drinks enhance colorectal cancer metastasis via SORD
The consumption of sugar-sweetened beverages (SSBs), which contain high levels of fructose and glucose, has been causally and mechanistically linked to an increased risk of colorectal cancer (CRC). However, the effects of SSB consumption on advanced stages of disease progression, including metastasis, remain poorly understood. Here we show that exposure of CRC cells to a glucose and fructose formulation—reflecting the composition of both high-fructose corn syrup and sucrose found in SSBs—enhances cellular motility and metastatic potential compared to glucose alone. Given that CRC cells grow poorly in fructose alone, and cells in vivo are not physiologically exposed to fructose without glucose, we excluded the fructose-only condition from our studies unless needed as a control. Mechanistically, the combination of glucose and fructose elevates the NAD⁺/NADH ratio by activation of the reverse reaction of sorbitol dehydrogenase in the polyol pathway. This redox shift relieves NAD⁺ limitations and accelerates glycolytic activity, which in turn fuels activation of the mevalonate pathway, ultimately promoting CRC cell motility and metastasis. Our findings highlight the detrimental impact of SSBs on CRC progression and suggest potential dietary and therapeutic strategies to mitigate metastasis in patients with CRC. Feng, Luo and colleagues show that the combination of glucose and fructose, as found in sugar-sweetened beverages, promotes colorectal cancer metastasis through a mechanism involving sorbitol dehydrogenase (SORD).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


