通过简单改性引入多个柔性氢键制备韧性弹性体
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
在聚合物中引入多个氢键(h键)作为动态交联可以增加韧性并实现动态功能。在这项工作中,我们报告了一种简单而通用的方法,将一种新的柔性氢键基团,即邻氨基醇(VAA)引入到市售的二烯基聚合物中。我们发现结构简单和灵活的氢键基团可以作为有效的动态交联,在不牺牲动态性能的情况下赋予机械稳健性。通过将聚丁二烯环氧化,再与多种胺进行开环反应,合成了vaa改性聚丁二烯。用邻苯二甲酸乙胺醇改性的PB在断裂时的应力增加了约90倍,韧性增加了约75倍。VAA改性使PB具有与共价交联弹性体相当的机械强度,同时保持了自恢复等动态性能。量子化学计算表明,VAA采用多种稳定的氢键二聚体结构,使二聚体在焓和熵上都保持稳定。此外,该方法也适用于其他二烯聚合物,如聚苯乙烯-嵌段聚丁二烯-嵌段聚苯乙烯。这项研究为利用柔性氢键的独特特性将各种二烯基聚合物转化为坚固而动态的高性能材料铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
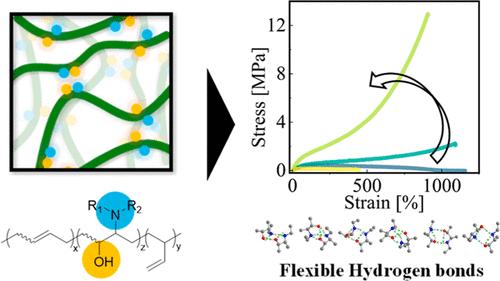
Preparation of a Tough Elastomer by the Introduction of Multiple Flexible Hydrogen Bonds via Simple Modification
The introduction of multiple hydrogen bonds (H-bonds) into polymers as dynamic cross-links can increase toughness and enable dynamic functions. In this work, we report a simple and versatile method to introduce a novel flexible H-bond group, namely, vicinal amino alcohol (VAA), into commercially available diene-based polymers. We found that structurally simple and flexible H-bonding groups can act as effective dynamic cross-links, conferring mechanical robustness without sacrificing dynamic properties. These VAA-modified polybutadiene (PB) were synthesized by the epoxidation of PB followed by a ring-opening reaction with various amines. PB modified with vicinal (ethylamino)alcohol presented approximately a 90-fold increase in stress at break and approximately a 75-fold increase in toughness. The VAA modification endowed PB with mechanical strength comparable to that of covalently cross-linked elastomers while maintaining dynamic properties such as self-recoverability. Quantum chemical calculations revealed that VAA adopted a wide variety of stable H-bonded dimer structures, which stabilized the dimer both enthalpically and entropically. Moreover, this method is applicable to other diene polymers, such as polystyrene-block-polybutadiene-block-polystyrene. This study paves the way to transform various diene-based polymers into robust yet dynamic high-performance materials by utilizing the unique features of flexible H-bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


