茴香酸通过E3连接酶募集促进hpv16e6的蛋白酶体降解:一项基于质谱的相互作用组研究。
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
人乳头瘤病毒(HPV)是宫颈癌和其他上皮癌的主要驱动因素,病毒癌蛋白E6通过促进肿瘤抑制因子p53的降解在肿瘤发生中发挥核心作用。虽然预防性疫苗可以预防感染,但仍然迫切需要消除已建立的hpv阳性细胞的治疗策略。本研究发现,天然二萜异戊酸(AA)作为一种新的药理学原理,可选择性诱导hpv16e6的降解。通过细胞热移分析,我们证明AA直接与E6相互作用,可能引发构象变化,促进其泛素化。对aa处理细胞中E6相互作用组的蛋白质组学分析显示,E3泛素连接酶(包括E6AP、UBR4、CDC20和TRIP12)以及蛋白酶体亚基持续富集。据我们所知,这代表了小分子处理条件下hpv16e6相互作用组的第一个全面的蛋白质组学框架。这些发现支持了AA促进蛋白酶体介导的E6消除的模型,并且该数据集本身为HPV生物学和治疗开发提供了及时和有价值的资源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
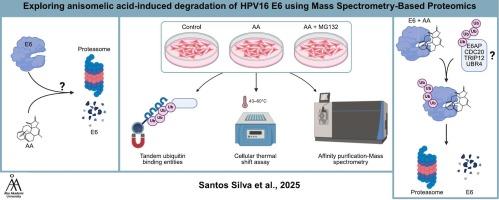
Anisomelic acid promotes proteasomal degradation of HPV16 E6 via E3 ligase recruitment: A mass spectrometry-based interactome study
Human papillomavirus (HPV) is a major driver of cervical and other epithelial cancers, with the viral oncoprotein E6 playing a central role in tumorigenesis by promoting degradation of the tumor suppressor p53. While prophylactic vaccines prevent infection, there remains a critical need for therapeutic strategies that eliminate established HPV-positive cells. Here, we identify anisomelic acid (AA), a natural diterpenoid, as a novel pharmacological principle that selectively induces the degradation of HPV16 E6. Using cellular thermal shift assay, we demonstrate that AA directly interacts with E6, likely triggering a conformational change that promotes its ubiquitination. Proteomic analysis of the E6 interactome in AA-treated cells revealed consistent enrichment of E3 ubiquitin ligases, including E6AP, UBR4, CDC20, and TRIP12, as well as proteasomal subunits. To our knowledge, this represents the first comprehensive proteomics framework of the HPV16 E6 interactome under small-molecule treatment conditions. These findings support a model in which AA facilitates proteasome-mediated elimination of E6, and the dataset itself provides a timely and valuable resource for HPV biology and therapeutic development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


