调节单胺能系统:3,5-二甲基-1-苯基-4-(苯硒基)- 1h -吡唑在脂多糖诱导的小鼠抑郁样症状中的研究。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究通过体内、体外和计算机方法研究了3,5-二甲基-1-苯基-4-(苯硒基)- 1h -吡唑(SePy)抗抑郁样药物的潜力,并评估了其肝脏和肾脏安全性。雄性瑞士小鼠接受脂多糖(LPS 0.83 mg/kg,腹腔注射)和SePy (10 mg/kg, ig)治疗。24小时后。采用悬尾试验(TST)和强迫游泳试验(FST)评估抗抑郁样活性。随后在前额皮质、海马和小肠中测量单胺氧化酶(MAO)活性,以研究以前未探索的机制途径。SePy显著减少了两项测试(TST和FST)的静止时间,并抑制了所有分析区域的MAO-A和MAO-B活性。硅对接表明SePy对两种MAO同工异构体的催化残基具有高亲和力,与参考抑制剂异羧化肼相当。毒理学分析显示,雌性小鼠的SePy (5-300 mg/kg)没有明显的肝脏改变(丙氨酸和天冬氨酸转氨酶),尽管尿素和肌酐水平升高表明高剂量可能对肾脏产生影响。综上所述,结果表明SePy可能通过单胺能调节发挥抗抑郁样作用,并显示出良好的肝脏安全性。需要进一步的研究来确认其肾脏安全性,并进一步探索其在炎症条件下的药理潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
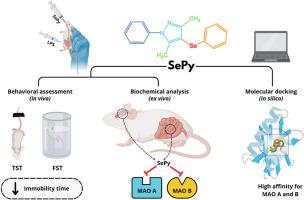
Modulating monoaminergic systems: A study on 3,5-dimethyl-1-phenyl-4-(phenylselenyl)-1H-pyrazole in lipopolysaccharide-induced depressive-like symptoms in mice
This study investigated 3,5-dimethyl-1-phenyl-4-(phenylselenyl)-1H-pyrazole (SePy) antidepressant-like potential using in vivo, ex vivo, and in silico approaches and evaluated its hepatic and renal safety profile. Male Swiss mice received lipopolysaccharide (LPS 0.83 mg/kg, intraperitoneally) and were treated with SePy (10 mg/kg, i.g.) 24 h later. Antidepressant-like activity was evaluated using the tail suspension test (TST) and forced swimming test (FST). Monoamine oxidase (MAO) activity was subsequently measured in the prefrontal cortex, hippocampi, and small intestine to investigate previously unexplored mechanistic pathways. SePy significantly reduced immobility time in both tests (TST and FST) and inhibited MAO-A and MAO-B activities in all regions analyzed. In silico docking indicated high affinity of SePy for catalytic residues of both MAO isoforms, comparable to the reference inhibitor isocarboxazide. Toxicological analysis of SePy (5–300 mg/kg) in female mice revealed no significant hepatic alterations (alanine and aspartate aminotransferase), although elevated urea and creatinine levels suggest possible renal effects at higher doses. Taken together, the results suggest that SePy may exert antidepressant-like effects via monoaminergic modulation and displays a favorable hepatic safety profile. Additional studies are warranted to confirm its renal safety and further explore its pharmacological potential under inflammatory conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


