在受损的人类神经祖细胞中恢复一氧化氮/过氧亚硝酸盐平衡:纳米医学方法及其对神经退行性疾病治疗的潜在影响
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
一氧化氮(NO)是一种重要的无机信号分子,参与了许多生理过程,并具有良好的治疗潜力。其氧化产物过氧亚硝酸盐(ONOO¯)具有细胞毒性,ONOO¯水平升高会引起氮氧化应激,这是神经退行性疾病(如阿尔茨海默病(AD))发病的一个因素。通过对人神经祖细胞(hNPCs)中NO和ONOO¯水平的药理调节,本研究探索了针对NO和神经元一氧化氮合酶(nNOS)通路(NO/nNOS)的潜在药物干预,通过恢复[NO]/[ONOO¯]平衡来预防或减少AD的进展。为了实现这一目标,金属卟啉纳米传感器被有效地用于实时、原位测量NO和ONOO¯浓度(200-300 nm直径),并精确定位在离hNPCs膜4-5±1 μm处,从而能够精确地研究[NO]/[ONOO¯]比率。[NO]/[ONOO¯]比值成为评估nNOS偶联/解耦与hNPC功能/功能障碍的关键生物标志物。在健康细胞中,这一比例约为0.25±0.005,而功能失调的hNPCs经淀粉样蛋白β42 (a - β42)处理后,这一比例急剧下降94%,表明严重的细胞功能障碍。基于这些发现,人们提出了通过恢复[NO]/[ONOO¯]平衡来预防或减少AD进展的潜在药物干预措施。值得注意的是,将一氧化氮合成的辅助因子前体sepapterin (SEP)与一种NADPH氧化酶抑制剂VAS 2870共同处理后,该比值部分恢复至0.1,表明nNOS功能得到改善。本文章由计算机程序翻译,如有差异,请以英文原文为准。
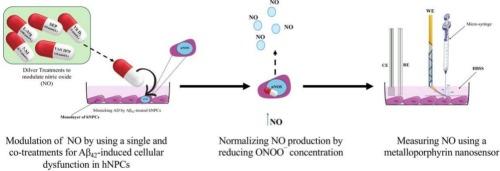
Restoring nitric oxide/Peroxynitrite equilibrium in impaired human neural progenitor cells: Nanomedical approaches and their potential impact on neurodegenerative disease treatment
Nitric oxide (NO), an essential inorganic signaling molecule, involved in many physiological processes and has promising therapeutic potential Its oxidation product, peroxynitrite (ONOO¯), is cytotoxic, and elevated ONOO¯ levels induce nitroxidative stress, a factor implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer's disease (AD). Through pharmacological modulation of NO and ONOO¯ levels in human neural progenitor cell (hNPCs), this study explores the potential pharmaceutical interventions targeting the NO and the neuronal nitric oxide synthase (nNOS) pathway, (NO/nNOS), to prevent or reduce AD progression by restoring the [NO]/[ONOO¯] balance. To achieve this, metalloporphyrin nanosensors have been effectively employed for real-time, in-situ measurement of NO and ONOO¯ concentrations (200–300 nm diameter) were applied and precisely positioned 4–5 ± 1 μm from hNPCs membranes, enabling precise investigation of the [NO]/[ONOO¯] ratio. The [NO]/[ONOO¯] ratio emerged as a critical biomarker for the evaluation of nNOS coupling/uncoupling to the hNPC functioning/dysfunction. In healthy cells, this ratio was around 0.25 ± 0.005, While dysfunctional hNPCs treated to amyloid beta 42 (Aβ42)—a hallmark of AD—caused a dramatic 94 % drop, signaling severe cellular dysfunction. Based on these findings, potential pharmacological interventions have been proposed to prevent or reduce AD progression by restoring the [NO]/[ONOO¯] balance. Notably, a co-treatment of sepiapterin (SEP), a cofactor precursor for NO synthesis, with VAS 2870 (an NADPH oxidase inhibitor) partially restored the ratio to 0.1, indicating improved nNOS function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


