基于喹唑啉的第四代EGFR酪氨酸激酶抑制剂克服非小细胞肺癌(NSCLC)中c797s介导的耐药
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
表皮生长因子受体酪氨酸激酶(EGFR-TK)耐药突变,特别是C797S的出现,极大地限制了奥西替尼治疗非小细胞肺癌(NSCLC)的长期疗效。在本研究中,我们设计并评估了一系列针对EGFR (L858R/T790M/C797S)三重突变体的喹唑啉衍生物。其中,化合物8d对EGFR L858R/T790M/C797S的效价最高,IC₅0为0.068 μM,与奥西替尼相比,具有较强的结合亲和力和有效的激酶活性抑制作用。分子对接研究揭示了与催化剂Lys745的关键相互作用。100 ns以上的分子动力学(MD)模拟证实了配体的稳定性,平均均方根偏差(RMSD)低于2.0 Å,结合自由能为-44 kcal/mol (MM/GBSA)。构效关系(SAR)分析表明,在喹唑啉环的C2位置,一个较大的疏水取代基与一个磺酰基结合,可以提高亲和性和效价。这些研究结果表明,喹唑啉衍生物,特别是化合物8d,有望成为克服非小细胞肺癌治疗中c797s介导的耐药的第四代EGFR抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
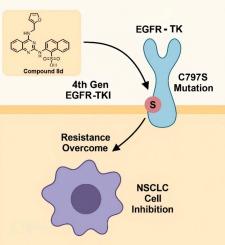
Quinazoline-based fourth-generation EGFR tyrosine kinase inhibitors to overcome C797S-mediated resistance in non-small cell lung Cancer (NSCLC)
The emergence of resistance mutations, particularly C797S, in epidermal growth factor receptor tyrosine kinase (EGFR-TK) has significantly limited the long-term efficacy of Osimertinib in non-small cell lung cancer (NSCLC). In this study, we designed and evaluated a series of quinazoline derivatives targeting the triple mutant EGFR (L858R/T790M/C797S). Among them, compound 8d exhibited the highest potency against EGFR L858R/T790M/C797S, with an IC₅₀ of 0.068 μM, demonstrating strong binding affinity and effective suppression of kinase activity compared to Osimertinib. Molecular docking studies revealed key interactions with catalytic Lys745. Molecular dynamics (MD) simulations over 100 ns confirmed ligand stability, with an average root-mean-square deviation (RMSD) below 2.0 Å and a binding free energy of −44 kcal/mol (MM/GBSA). Structure-activity relationship (SAR) analysis highlighted the critical role of a bulkier hydrophobic substituent at the C2 position of the quinazoline ring in combination with a sulfonyl group, which improved affinity and potency. These findings establish quinazoline derivatives, particularly compound 8d, as promising fourth-generation EGFR inhibitors for overcoming C797S-mediated resistance in NSCLC therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


