从两个海洋分离的青霉中分离出的Sirenin和bisabolane倍半萜类通过调节nod样受体信号通路具有抗骨质疏松活性。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从从海洋红藻翼藻(Pterocladiella tenuis)和海洋石鳖(Liolophura japonica)中分离得到的2株海洋真菌青霉EN-480和as -400中分离得到9个未报道的(chermeisabolins A- i, 1-9)和1个已知的(eupenicisirenin C, 10) sirenin倍半萜类化合物和2个未报道的双甾烷型倍半萜类化合物(chermebisabolins A和B, 11和12)。sirenin倍半萜类化合物(1-9)具有独特的6/3双环体系,化合物8和9通过降解C-12甲基而成为非sirenin类似物。通过核磁共振解释、ECD分析、Mosher法、x射线晶体衍射和量子化学计算确定了它们的结构,包括立体化学构型。斑马鱼骨质疏松模型的抗骨质疏松活性评价显示,切美西甲素A(1)能显著缓解强的松龙治疗的斑马鱼骨荧光面积和荧光密度的减少。此外,化合物1可以下调强龙诱导的破骨细胞发生相关基因ctsk和tnfrsf11b的表达,改善骨形成状态。通过转录组学分析探索其机制发现,化合物1可能作用于参与炎症反应和骨代谢调节的nod样受体(NLR)信号通路,这在以往的倍半萜类研究中未见报道。这些结果表明,化合物1作为治疗骨质疏松症的潜在候选物值得进一步开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
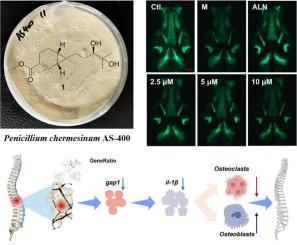
Sirenin and bisabolane sesquiterpenoids from two marine isolates of Penicillium chermesinum with anti-osteoporotic activity by modulating NOD-like receptor signaling pathway
Nine unreported (chermesirenins A–I, 1–9) and one known (eupenicisirenin C, 10) sirenin sesquiterpenoids as well as two unreported bisabolane-type sesquiterpenoids (chermebisabolins A and B, 11 and 12) were isolated from two isolates of marine-sourced fungal strains Penicillium chermesinum EN-480 and AS-400, which were obtained as endophytic and endozoic fungi from the marine red alga Pterocladiella tenuis and the marine chiton Liolophura japonica, respectively. The sirenin sesquiterpenoids (1–9) featured with unique 6/3 dicyclic ring system while compounds 8 and 9 are nor-sirenin analogs by degrading the C-12 methyl group. Their structures including stereochemical configurations were confirmed through NMR interpretation, ECD analysis, Mosher's method, X-ray crystallographic diffraction, and quantum chemical calculations. The anti-osteoporotic activity evaluation in the zebrafish osteoporosis model revealed that chermesirenin A (1) could significantly alleviate the reduction of bone fluorescence area and fluorescence density in prednisolone-treated zebrafish. Furthermore, compound 1 could down-regulate the expression of the genes associated with prednisolone-induced osteoclastogenesis, ctsk and tnfrsf11b, to improve bone formation status. Exploration on the mechanism through the transcriptomic analysis showed that compound 1 might act on the NOD-like receptor (NLR) signaling pathway involved in inflammatory responses and bone metabolism regulation, which has not been reported among previous investigations on sesquiterpenoids. These results demonstrated that compound 1 deserved further development as a potential candidate towards the therapy of osteoporotic disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


