奥贝胆酸通过肝细胞介导的HMGB1释放,在胆汁淤积中诱导溶酶体膜渗透,从而加重肝损伤。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
奥贝胆酸(OCA)是一种治疗对熊去氧胆酸(UDCA)无反应的原发性胆管炎(PBC)的药物,具有肝毒性风险,可能加重肝功能衰竭并增加死亡率。关于OCA的安全性研究是指导临床实践的必要条件。高迁移率组盒1 (HMGB1)是一种与多种肝脏病理有关的细胞外损伤相关的分子模式分子,在oca诱导的肝损伤背景下进行了机制研究。在胆管结扎(BDL)小鼠中,OCA (40mg/kg)显著升高血清和细胞质HMGB1水平,与胆管反应、坏死和肝纤维化加剧相关。丙酮酸乙酯对HMGB1的药理抑制减轻了这些病理特征。在体外,OCA (100 μM)刺激原代肝细胞上清液中HMGB1的释放(~ 100 pg/mL)。机制研究表明,oca诱导的溶酶体膜通透性引发胞质酸蛋白酶渗漏,阻止胞质HMGB1的自噬降解,促进其随后的胞外释放。这些发现表明,oca诱导的肝细胞死亡是通过溶酶体膜渗透发生的,这随后导致HMGB1的释放。这突出了HMGB1或溶酶体功能作为减轻oca相关肝毒性的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
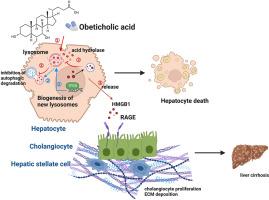
Obeticholic acid exacerbates liver injury through hepatocyte-mediated release of HMGB1 by inducing lysosomal membrane permeabilization in cholestasis
Obeticholic acid (OCA), a therapy for primary biliary cholangitis (PBC) unresponsive to ursodeoxycholic acid (UDCA), poses risks of hepatotoxicity that may worsen liver failure and increase mortality. Safety studies regarding OCA are imperative for guiding clinical practice. High-mobility group box 1 (HMGB1), an extracellular damage-associated molecular pattern molecule implicated in various liver pathologies, was mechanistically examined in the context of OCA-induced liver injury. In bile duct ligation (BDL) mice, OCA (40 mg/kg) administration significantly elevated serum and cytoplasmic levels of HMGB1, correlating with an exacerbated ductular reaction, necrosis, and liver fibrosis. Pharmacological inhibition of HMGB1 using ethyl pyruvate alleviated these pathological features. In vitro, OCA (100 μM) stimulated the release of HMGB1 (∼100 pg/mL) from primary hepatocyte supernatants. Mechanistic studies revealed that OCA-induced lysosomal membrane permeabilization triggered the leakage of cytosolic acid proteases, which prevented the autophagic degradation of cytoplasmic HMGB1 and promoted its subsequent extracellular release. These findings demonstrate that OCA-induced hepatocyte cell death occurs through lysosomal membrane permeabilization, which subsequently leads to the release of HMGB1. This highlights HMGB1 or lysosomal function as potential therapeutic targets for mitigating OCA-associated hepatotoxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


