茶黄素3,3'-二二酸抑制黑色素瘤细胞转移并降低胰岛素样生长因子-1诱导的肿瘤干性和侵袭性
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
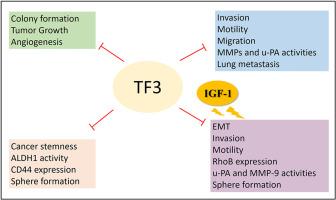
茶黄素3,3'-二allate (TF3)是一种类黄酮化合物,具有抗糖尿病作用,诱导细胞凋亡,抑制肿瘤生长。然而,其对黑色素瘤转移和胰岛素样生长因子1 (IGF-1)诱导的癌症干细胞的影响在很大程度上仍未被探索。本研究探讨了TF3在黑色素瘤中抗肿瘤和抗转移活性的关键分子信号通路。TF3的抗转移潜能,以及它对igf -1诱导的癌症干细胞的影响,通过伤口愈合实验、明胶酶谱、Matrigel侵袭实验、球形成实验和Western blotting来评估。通过皮下移植和尾静脉注射小鼠模型进一步检测其抗血管生成和抗转移活性。TF3显著抑制a375和A2058黑色素瘤细胞的细胞迁移、侵袭和基质金属蛋白酶(MMP)活性。它还抑制球的形成、自我更新能力和醛脱氢酶1 (ALDH1)的活性。在球形黑色素瘤细胞中,TF3下调关键的癌症干细胞和耐药标志物,包括ABCB1、ABCG2、CD44和CXCR4。值得注意的是,TF3逆转了igf -1诱导的球体形成、侵袭和MMP-9的表达。在机制上,TF3抑制igf -1介导的局灶黏附激酶(FAK)和Src的磷酸化。在体内,TF3显著抑制肿瘤生长,降低血管生成标志物表达,抑制肺转移。总之,这些发现表明TF3通过抑制转移和抑制igf -1诱导的癌症干性和侵袭性来阻碍黑色素瘤的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theaflavin 3,3′-digallate suppresses metastasis and reduces insulin-like growth factor-1-induced cancer stemness and invasiveness in human melanoma cells
Theaflavin 3,3′-digallate (TF3), a flavonoid compound, exerts antidiabetic effects, induces apoptosis, and suppresses cancer growth. However, its effects on melanoma metastasis and insulin-like growth factor 1 (IGF-1)-induced cancer stemness remain largely unexplored. This study explored key molecular signaling pathways underlying the antitumor and antimetastatic activities of TF3 in melanoma. The antimetastatic potential of TF3, as well as its impact on IGF-1-induced cancer stemness, were evaluated using wound healing assays, gelatin zymography, Matrigel invasion assays, sphere formation assays, and Western blotting. Its antiangiogenic and antimetastatic activities were further examined in vivo using subcutaneous xenograft and tail vein injection mouse models. TF3 significantly inhibited cell migration, invasion, and matrix metalloproteinase (MMP) activity in A 375 and A2058 melanoma cells. It also suppressed sphere formation, self-renewal capacity, and aldehyde dehydrogenase 1 (ALDH1) activity. In sphere-forming melanoma cells, TF3 downregulated key cancer stemness and drug resistance markers, including ABCB1, ABCG2, CD44, and CXCR4. Notably, TF3 reversed IGF-1-induced sphere formation, invasion, and MMP-9 expression. Mechanistically, TF3 suppressed IGF-1-mediated phosphorylation of focal adhesion kinase (FAK) and Src. In vivo, TF3 significantly inhibited tumor growth, reduced angiogenic marker expression, and suppressed lung metastasis. Collectively, these findings demonstrate that TF3 impedes melanoma progression by inhibiting metastasis and suppressing IGF-1-induced cancer stemness and invasiveness.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


