羟基胆固醇通过质膜胆固醇重塑加剧肝脏胰岛素抵抗。
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
背景和目的:胰岛素抵抗是代谢紊乱的关键驱动因素,但其分子机制尚不明确。本研究确定了27-羟基胆固醇(27HC),一种胆固醇衍生的代谢物,并研究了其在胰岛素抵抗中的作用。方法:靶向代谢组学量化患者、小鼠和肝细胞中27HC (27HC/胆固醇比值)的绝对和相对水平。建立胰岛素抵抗小鼠模型,表征27HC及相关酶的时空动态。功能分析评估了27HC对多种肝细胞类型胰岛素信号传导的影响。转录组学分析确定了关键的效应通路。用生物传感器评估质膜胆固醇可及性,并通过胆固醇抢救进行验证。采用膜蛋白提取、免疫荧光和流式细胞术评估27HC对胰岛素受体(IR)分布和结合能力的影响。结果:在代谢功能障碍相关脂肪变性肝病(MASLD)、肥胖和2型糖尿病小鼠(T2DM)以及pa处理的HepG2和原代肝细胞中观察到27HC水平升高,与胰岛素敏感性受损相关。CYP27A1被认为是调节肝脏27HC水平的关键酶。体外研究表明,27HC破坏HepG2、AML12和原代肝细胞中的胰岛素信号传导,而CYP27A1敲低可恢复IR反应性。27HC抑制srebp2依赖性胆固醇的生物合成,耗尽质膜内可接近的胆固醇,引发IR错定位和信号衰减。高脂饮食小鼠肝脏特异性CYP27A1沉默可改善全身胰岛素敏感性并恢复代谢稳态。结论:我们的研究结果表明,27HC是连接胆固醇代谢和胰岛素抵抗的关键效应因子,并提出CYP27A1抑制是胰岛素抵抗的潜在治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
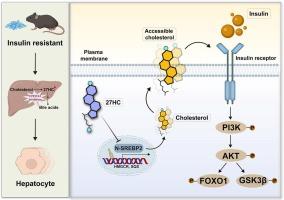
27-Hydroxycholesterol exacerbates hepatic insulin resistance via plasma membrane cholesterol remodeling
Background and aims
Insulin resistance is a key driver of metabolic disorders, yet its molecular mechanisms remain elusive. This study identifies 27-hydroxycholesterol (27HC), a cholesterol-derived metabolite, and investigates its role in insulin resistance.
Methods
Targeted metabolomics quantified absolute and relative levels of 27HC (27HC/cholesterol ratio) in patients, mice, and hepatocytes. Insulin resistant mouse models were established to characterize spatiotemporal dynamics of 27HC and related enzymes. Functional analyses assessed 27HC's effect on insulin signaling across multiple hepatocyte types. Transcriptomic analysis identified key effector pathways. Plasma membrane cholesterol accessibility was evaluated using biosensors and validated by cholesterol rescue. Membrane protein extraction, immunofluorescence, and flow cytometry were employed to assess the impact of 27HC on insulin receptor (IR) distribution and binding capacity.
Results
Elevated 27HC levels were observed in patients with metabolic dysfunction-associated steatotic liver disease (MASLD), obese and type 2 diabetic mice (T2DM), and PA-treated HepG2 and primary hepatocytes, correlating with impaired insulin sensitivity. CYP27A1 was identified as the key enzyme regulating liver 27HC levels. In vitro studies demonstrated that 27HC disrupts insulin signaling in HepG2, AML12, and primary hepatocytes, whereas CYP27A1 knockdown restored IR responsiveness. 27HC suppresses SREBP2-dependent cholesterol biosynthesis, depleting accessible cholesterol in the plasma membrane, triggering IR mislocalization and signal attenuation. Liver-specific CYP27A1 silencing in mice fed a high-fat diet improved systemic insulin sensitivity and restored metabolic homeostasis.
Conclusion
Our findings establish 27HC as a key effector linking cholesterol metabolism to insulin resistance and propose CYP27A1 inhibition as a potential therapeutic strategy for insulin resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


