ATAD3A缺乏通过复合体I逆向电子传递诱导氧化应激。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
基于氧化还原稳态的重要性,精确氧化还原调控的概念受到了广泛关注。作为活性氧(Reactive Oxygen Species, ROS)产生的主要来源,线粒体在这一过程中起着双重作用:促进有益的信号传递和造成过度的氧化损伤。大量研究表明,这种二元性不仅取决于产生活性氧的数量,而且取决于产生活性氧的不同位点,每个位点都有不同的影响。这一见解强调了准确调节线粒体氧化还原的必要性和重要性。然而,具体的监管体系仍不清楚。在我们的研究中,我们发现特异性敲除atad-3 (ATAD3A)基因可显著增加线虫和哺乳动物细胞中线粒体ROS的水平。我们发现ATAD3A直接与复合物I亚基NDUFS8相互作用,在复合物I的组装和活性中起着不可或缺的作用。降低atad-3降低复合物I活性和质子泄漏,增加线粒体膜电位,从而诱导反向电子传递(RET)产生更多ROS。诱导的RET-ROS可能作为线粒体功能受损的保护性反应,激活抗氧化系统,增强线虫的抗逆性并延长寿命。我们的研究结果揭示了一种新的功能和机制,即敲低ATAD3A以精确调节线粒体ROS的产生。他们为RET-ROS作为促进寿命的信号的有益作用提供了证据,并强调了精确氧化还原的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
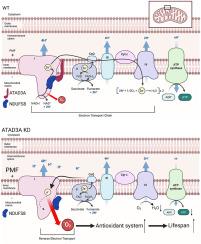
ATAD3A deficiency induces oxidative eustress via the complex I reverse electron transport
Based on the importance of redox homeostasis, the concept of precision redox regulation has received widespread attention. As the main source of Reactive Oxygen Species (ROS) production, mitochondria play a dual role in this process: facilitating beneficial signal transmission and causing excessive oxidative damage. Numerous studies have demonstrated that this duality is not only dependent on the quantity of ROS produced but also on the different sites of production, each showing varying effects. This insight underscores the necessity and importance of accurately regulating mitochondrial redox. However, the precise regulatory system remains unclear. In our study, we discovered that specifically knocking down the atad-3 (ATAD3A) gene significantly increased the level of mitochondrial ROS in nematodes and mammalian cells. We found that ATAD3A directly interacts with the complex I subunit NDUFS8, playing an integral role in complex I assembly and activity. Knocking down atad-3 reduces complex I activity and proton leakage, increases mitochondrial membrane potential, thereby inducing reverse electron transport (RET) to produce more ROS. The induced RET-ROS may serve as a protective response to impaired mitochondrial function, activating antioxidant systems that enhance stress resistance and extend longevity in nematodes. Our findings reveal a novel function and mechanism of knocking down ATAD3A to precisely regulate mitochondrial ROS production. They provide evidence for the beneficial role of RET-ROS as a signal promoting longevity and underscore the importance of precision redox.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


