选择性抑制PI3Kγ可减轻化疗诱导的肠黏膜炎而不影响伊立替康的抗癌特性。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
以伊立替康为基础的抗癌治疗可诱发肠黏膜炎,增加败血症和患者死亡的风险。磷酸肌肽3-激酶(PI3K)参与癌症和炎症性疾病,但其在肠黏膜炎中的作用尚不清楚。C57BL/6雄性小鼠分别给药(1% DMSO, 10 ml/kg, p.o)、伊立替康单独或与AS-605240 (PI3Kγ抑制剂,10 mg/kg, p.o)或GSK2269557 (PI3Kδ抑制剂,3 mg/kg, p.o)联合。通过组织病理学、炎症标志物和腹泻来评估黏膜炎。用mc38结直肠癌细胞系接种小鼠,观察其抗肿瘤效果。PI3Kγ抑制可减轻伊立替康诱导的肠道损伤,这可以通过改善绒毛/隐窝比来证明。PI3Kγ抑制还导致中性粒细胞的轻度积累,Tlr2、Tlr4和Tlr9的表达减少,白细胞介素-1β和-6的水平降低,巨噬细胞标志物F4/80和调节性T细胞转录因子FOXP3 (P本文章由计算机程序翻译,如有差异,请以英文原文为准。
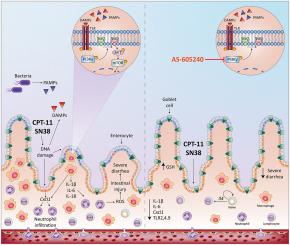
Selective PI3Kγ inhibition attenuates chemotherapy-induced intestinal mucositis without compromising the anticancer properties of irinotecan
Irinotecan-based anticancer therapy induces intestinal mucositis, increasing the risk of sepsis and patient death. The phosphoinositide 3-kinases (PI3K) are involved in cancer and inflammatory diseases, but their role in intestinal mucositis remains unknown. C57BL/6 male mice received vehicle (1 % DMSO, 10 ml/kg, p.o.), irinotecan alone or in combination with AS-605240 (a PI3Kγ inhibitor, 10 mg/kg, p.o.) or GSK2269557 (a PI3Kδ inhibitor, 3 mg/kg, p.o.). Histopathology, inflammatory markers, and diarrhea were assessed to evaluate mucositis. The antitumor effect was evaluated in mice inoculated with the Mc-38 colorectal cancer cell line. PI3Kγ inhibition attenuated irinotecan-induced intestinal injury, as evidenced by improved villus/crypt ratio. PI3Kγ inhibition also caused milder neutrophil accumulation, reduced expression of Tlr2, Tlr4, and Tlr9, and decreased levels of interleukin-1β and −6, as well as attenuated immunofluorescence for F4/80, a macrophage marker, and the regulatory T cell transcription factor FOXP3 (P < 0.05 vs. irinotecan). Additionally, AS-605240 prevented goblet cell loss and attenuated diarrhea. Moreover, PI3Kγ inhibition combined with irinotecan showed no synergistic anticancer effects. In contrast, PI3Kδ blockade did not prevent the development of mucositis but rather enhanced neutrophil accumulation in the intestine. PI3Kγ inhibition attenuates chemotherapy-associated intestinal mucositis without compromising the anticancer efficacy of irinotecan. However, selective inhibition of PI3Kδ exacerbates the inflammatory response and tissue damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


