浸润性巨噬细胞取代库普弗细胞在严重酒精相关性肝炎中发挥多种作用。
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
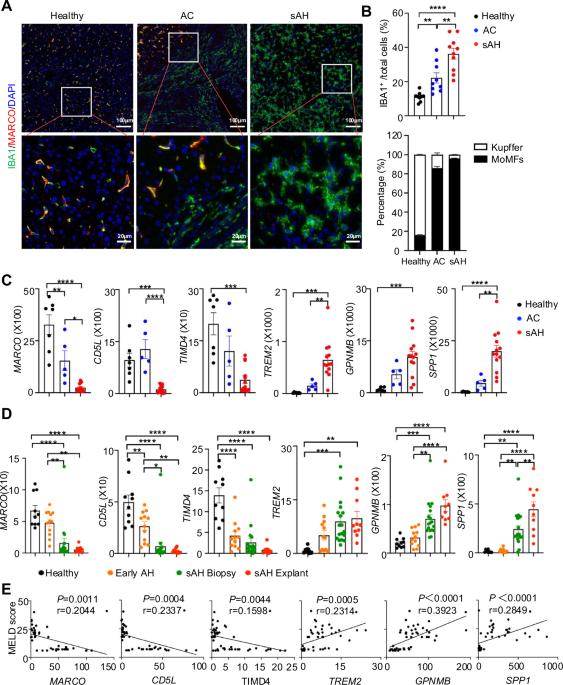
酒精相关性肝硬化(AC)患者可发展为严重酒精相关性肝炎(sAH),这是一种短期死亡率很高的疾病。我们之前的研究表明,sAH,而不是AC肝脏,被大量自我维持的IL-8+中性粒细胞浸润,这可能推动了从AC到sAH的转变。单核细胞来源的巨噬细胞(momf)也在sAH中浸润肝脏,但它们的作用在很大程度上仍然不清楚。在本研究中,我们对来自sAH和AC患者的人肝外植体中的肝巨噬细胞进行了表征。我们的数据显示Kupffer细胞显著减少,而在sAH和AC中momf增加。单细胞RNA-Seq分析显示AC和sAH中有几个群体,包括C1Q+、S100A8+、APOE+、TNF+和VSIG4+巨噬细胞,sAH中含有独特的C1Q+巨噬细胞,可能在去除sAH中凋亡的中性粒细胞中发挥作用。C1Q+巨噬细胞还表达了许多参与吞噬和促炎、抗炎功能的基因,提示C1Q+巨噬细胞在sAH中具有多种功能。在酒精性肝损伤实验模型中进一步研究C1Q、S100A8和APOE的作用。我们的数据显示,C1q KO小鼠和巨噬细胞特异性S100a8 KO小鼠表现出相似的酒精诱导的肝损伤和肝中性粒细胞浸润,而Apoe KO小鼠在慢性+暴饮乙醇刺激下发生的肝损伤比WT小鼠严重得多。综上所述,sAH和AC被多种巨噬细胞浸润,这些巨噬细胞发挥不同的功能,推动慢性疾病的进展。sAH中独特的C1Q+巨噬细胞在清除死细胞中起代偿作用,但也可能促进sAH的炎症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Infiltrating macrophages replace Kupffer cells and play diverse roles in severe alcohol-associated hepatitis
Patients with alcohol-associated cirrhosis (AC) may develop severe alcohol-associated hepatitis (sAH), a disease with high short-term mortality. Our previous studies demonstrated that sAH, but not AC livers, are infiltrated with a high number of self-sustaining IL-8+ neutrophils that likely drive the transition from AC to sAH. Monocyte-derived macrophages (MoMFs) also infiltrate the liver in sAH, but their roles remain largely obscure. In the present study, we characterized liver macrophages in human liver explants from sAH and AC patients. Our data revealed a marked reduction in Kupffer cells, whereas MoMFs were increased in sAH and AC. Single-cell RNA-Seq analyses revealed several populations in both AC and sAH, including C1Q+, S100A8+, APOE+, TNF+ and VSIG4+ macrophages, with sAH containing unique C1Q+ macrophages potentially playing a role in removing apoptotic neutrophils in sAH. C1Q+ macrophages also express many genes involved in phagocytosis and proinflammatory and anti-inflammatory functions, suggesting that C1Q+ macrophages have diverse functions in sAH. The roles of C1Q, S100A8, and APOE were further examined in experimental models of alcohol-induced liver injury. Our data revealed that C1q KO mice and macrophage-specific S100a8 KO mice presented similar alcohol-induced liver injury and hepatic neutrophil infiltration, while Apoe KO mice developed much more severe liver injury than did WT mice following chronic-plus-binge ethanol challenge. Taken together, sAH and AC are infiltrated with multiple populations of macrophages that perform diverse functions to drive chronic disease progression. Unique C1Q+ macrophages in sAH play a compensatory role in removing dead cells but may also promote inflammation in sAH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


