铜催化的hfip通过宝石二氟化环丙烷的C-C键激活促进了苯酚的对选择性烯丙基化
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
虽然使用过渡金属催化剂进行宝石-二氟环丙烷开环交叉偶联是一种探索得很好的策略,但开发具有成本效益的金属催化剂用于这种转化仍然不常见。本研究提出了通过开环交叉偶联方法实现铜催化的无保护酚的准选择性氟烯丙基化。这种简单的方法采用了一种廉价且容易获得的催化剂,提供了广泛的底物范围,并支持生物活性化合物的后期功能化。机理研究表明,烯丙基Cu(III)中间体是通过C-C键氧化加入Cu(I),然后C-F键消除而产生的。对选择性主要是由于六氟异丙醇与苯酚之间的氢键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
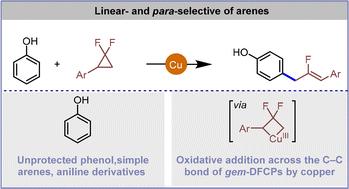
Copper-catalyzed HFIP-promoted para-selective allylation of phenols via C–C bond activation of gem-difluorinated cyclopropanes
Although ring-opening cross-coupling of gem-difluorocyclopropanes using transition metal catalysts is a well-explored strategy, the development of cost-effective metal catalysts for this transformation is still uncommon. This study presents a copper-catalyzed para-selective fluoroallylation of unprotected phenols achieved through a ring-opening cross-coupling approach. This straightforward method employs an inexpensive and easily accessible catalyst, offers a wide substrate scope, and supports late-stage functionalization of biologically active compounds. Mechanistic investigations suggest that an allyl–Cu(III) intermediate is generated through C–C bond oxidative addition to Cu(I), followed by C–F bond elimination. The para-selectivity is primarily attributed to hydrogen bonding between hexafluoroisopropanol and the phenol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


