肌因子介导的肌肉-器官相互作用:分子机制和临床意义。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
定期的运动训练通过协调的多器官相互作用来保持系统的内稳态,骨骼肌作为关键的效应器官和综合信号联系出现。最近的研究突破已经确定了骨骼肌的内分泌器官特性。通过收缩诱导的肌因子释放,骨骼肌采用包括自分泌、旁分泌和内分泌途径在内的多模态信号机制,系统地阐明了运动益处的分子基础。本文创新性地提出了“肌因子介导的多器官代谢网络”理论,全面总结了肌因子在介导器官间相互作用和动态通讯机制中的作用。这些相互作用涉及骨骼肌本身和多个重要器官,包括心、肝、肺、肾、胰腺、脑、骨、皮肤、口腔、脂肪组织、肠、胃、乳腺、卵巢和前列腺。机制研究阐明了肌因子作为多效信号调节剂的功能,在六个相互关联的生物轴上协调多方面的调控程序,包括能量底物通量和线粒体生物发生、成骨分化和细胞外基质重塑、神经可塑性和血脑屏障(BBB)稳态、肠道微生物群调节、血管内皮功能和肿瘤龛内的免疫代谢重编程。值得注意的是,肌因子的多靶点调控能力为运动诱导的疾病抵抗提供了机制见解。作为“运动模拟分子”,肌因子的靶向递送策略为代谢性疾病、神经退行性疾病和癌症治疗提供了新的治疗方向。至关重要的是,三个研究前沿需要优先考虑:解码时空肌因子分泌模式;绘制跨器官受体-配体相互作用网络;开发计算模型预测系统水平对肌因子调节的反应。解决这些挑战将促进运动生理学的发现转化为精确的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
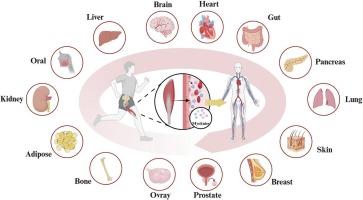
Myokine-mediated muscle-organ interactions: Molecular mechanisms and clinical significance
Regular exercise training preserves systemic homeostasis via coordinated multi-organ interactions, with skeletal muscle emerging as a pivotal effector organ and integrative signaling nexus. Recent breakthroughs in research have established the endocrine organ properties of skeletal muscle. Through contraction-induced release of myokines, skeletal muscle employs multimodal signaling mechanisms including autocrine, paracrine, and endocrine pathways, systematically elucidating the molecular basis of exercise benefits. This review innovatively proposes the “Myokine-mediated Multi-organ Metabolic Network” theory, comprehensively summarizing the role of myokines in mediating inter-organ crosstalk and dynamic communication mechanisms. These interactions involve skeletal muscle itself and multiple vital organs including heart, liver, lung, kidney, pancreas, brain, bone, skin, oral cavity, adipose tissue, intestine, stomach, mammary glands, ovaries, and prostate. Mechanistic insights elucidate that myokines function as pleiotropic signaling modulators, orchestrating multifaceted regulatory programs across six interconnected biological axes, including energy substrate flux and mitochondrial biogenesis, osteogenic differentiation and extracellular matrix remodeling, neuroplasticity and Blood-brain barrier (BBB) homeostasis, gut microbiota modulation, vascular endothelial function, and immunometabolic reprogramming within neoplastic niches. Notably, the multi-target regulatory capacity of myokines provides mechanistic insights into exercise-induced disease resistance. As “exercise-mimetic molecules,” targeted delivery strategies of myokines provide novel therapeutic directions for metabolic diseases, neurodegenerative disorders, and cancer treatment. Crucially, three research frontiers demand prioritization: decoding spatiotemporal myokine secretion patterns; mapping receptor-ligand interaction networks across organs; and developing computational models predicting system-level responses to myokine modulation. Addressing these challenges will catalyze the translation of exercise physiology discoveries into precision therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


