新型氨基酸取代和酰化spexin类似物对胰腺β细胞功能、食欲和葡萄糖稳态的影响。
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
目的:神经肽spexin被认为是一种诱导饱腹感的激素,但对新陈代谢的总体影响较少。快速的酶代谢意味着阐明spexin的生物效应是具有挑战性的,因为生物活性谱太短而无法确定。方法:因此,在本研究中,合成了Asn1取代spexin类似物D1Spx的Asp1,以及两个相关的脂肪酸衍生类似物(γGlu-Pal)-D1Spx和(K11γGlu-Pal)-D1Spx,并研究了对小鼠胰腺β细胞分泌功能和健康的影响,以及对食欲和葡萄糖稳态的影响。结果:初步证实了Spexin在BRIN-BD11 β细胞中的免疫反应性。有趣的是,与天然spexin一样,D1Spx容易被血浆酶降解,但脂肪酸衍生的分子保持完整。没有一种肽能增强BRIN-BD11细胞的胰岛素分泌。此外,spexin肽抑制丙氨酸诱导的胰岛素分泌,而天然spexin对细胞内钙没有影响。然而,所有spexin肽(10-8和10-6 M)促进β细胞增殖,而天然spexin和(γGlu-Pal)-D1Spx保护细胞因子诱导的β细胞凋亡。当小鼠腹腔注射spexin肽时,即使在250 nmol/kg的高剂量下,spexin肽也缺乏食欲调节作用。在联合注射生理盐水后,除了D1Spx可以忽略不计的增加外,spexin肽没有影响血糖水平。当与葡萄糖一起使用时,(γ - glu - pal)-D1Spx在注射后30分钟略有升高血糖,但与单独使用葡萄糖相比,spexin肽之间没有总体差异。结论:酰基化产生稳定的spexin类似物,其生物活性与天然spexin相似,包括促进β细胞增殖和部分保护细胞凋亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
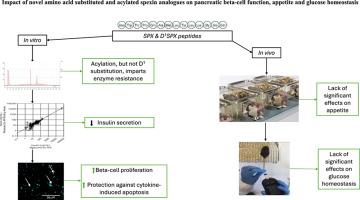
Impact of novel amino acid substituted and acylated spexin analogues on pancreatic beta-cell function, appetite and glucose homeostasis
Objective
The neuropeptide spexin is recognised as a satiety-inducing hormone, but overall effects on metabolism are less characterised. Rapid enzymatic metabolism means elucidating biological effects of spexin is challenging, because the bioactive profile is short.
Methods
Therefore, in the present study, an Asp1 for Asn1 substituted spexin analogue, D1Spx, alongside two related fatty acid derivatised analogues, (γGlu-Pal)-D1Spx and (K11γGlu-Pal)-D1Spx, were synthesised and effects on pancreatic beta-cell secretory function and health investigated together with impact on appetite and glucose homeostasis in mice.
Results
Spexin immunoreactivity was initially confirmed in BRIN-BD11 beta-cells. Interestingly, like native spexin, D1Spx was liable to plasma enzyme degradation, but the fatty acid derivatised molecules remained intact. None of the peptides augmented insulin secretion from BRIN-BD11 cells. Moreover, the spexin peptides inhibited alanine‐induced insulin secretion, with native spexin having no effect on intracellular calcium. However, all spexin peptides (10−8 and 10−6 M) promoted beta-cell proliferation, whilst native spexin and (γGlu-Pal)-D1Spx protected against cytokine-induced beta-cell apoptosis. When administered intraperitoneally to mice, spexin peptides lacked effects on appetite regulation, even at elevated doses of 250 nmol/kg. Following conjoint injection with saline, none of the spexin peptides affected blood glucose levels barring a negligible increase by D1Spx. When administered together with glucose, (γGlu-Pal)-D1Spx slightly increased blood glucose at 30 min post-injection, but there was no overall difference between the spexin peptides when compared to glucose alone.
Conclusions
Acylation creates stable spexin analogues with similar bioactivity as native spexin, including promotion of beta-cell proliferation and partial protection against apoptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


