“hipsc衍生的NSCs和神经元对百草枯治疗的易感性:对线粒体相关的不同神经毒性机制的见解。”
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
环境暴露于百草枯(PQ)是一种广泛使用的除草剂,与帕金森病等神经退行性疾病的风险增加有关。然而,传统动物模型的物种特异性限制阻碍了对人类神经毒性的机制见解。我们使用基于神经干细胞(NSCs)和来源于人诱导多能干细胞(hiPSCs)的神经元的人类相关细胞平台来研究PQ暴露后线粒体反应和细胞命运的差异。我们的研究结果表明,hipsc衍生的神经元对pq诱导的毒性的易感性明显高于相应的神经祖细胞。神经元易损的特征是线粒体膜深度去极化,线粒体质量减少,活性氧升高,一氧化氮水平升高,ATP产生减少,线粒体依赖的凋亡途径激活,包括caspase-9和caspase-3切割,同时BAX/BCL-XL比值升高。相比之下,hipsc衍生的NSCs通过上调糖酵解活性来维持生存,这可以通过GLUT-1表达和己糖激酶活性的增加来证明,这表明代谢适应支持对线粒体损伤的抵抗。值得注意的是,抗氧化剂n -乙酰- l-半胱氨酸部分恢复了hipsc来源的NSCs的线粒体膜电位和代谢,但未能保护神经元,突出了细胞类型特异性敏感性。线粒体动力学的改变,特别是神经元中OPA-1和MFN-2蛋白表达的减少,进一步支持线粒体结构和稳态的破坏。我们的研究强调了hipsc衍生的神经模型的翻译潜力,作为揭示PQ和其他与帕金森病风险相关的化学物质诱导的神经毒性机制的强大平台,以及揭示线粒体氧化应激的独特细胞反应。这些发现为早期发育过程中的神经元脆弱性提供了重要的见解,并为在神经退行性背景下有针对性地干预以保持线粒体完整性提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
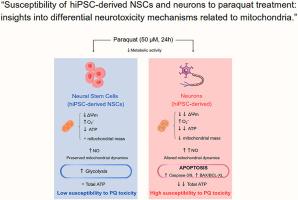
“Susceptibility of hiPSC-derived NSCs and neurons to paraquat treatment: insights into differential neurotoxicity mechanisms related to mitochondria.”
Environmental exposure to paraquat (PQ), a widely used herbicide, has been associated with an increased risk of neurodegenerative diseases such as Parkinson's disease. However, species-specific limitations of traditional animal models hinder mechanistic insights into human neurotoxicity. We used a human-relevant cellular platform based on neural stem cells (NSCs) and neurons derived from human induced pluripotent stem cells (hiPSCs) to investigate the differential mitochondrial response and cell fate following PQ exposure. Our results reveal that hiPSC-derived neurons exhibit markedly higher susceptibility to PQ-induced toxicity than their corresponding neural progenitor cells. The neuronal vulnerability is characterized by profound mitochondrial membrane depolarization, reduced mitochondrial mass, elevated reactive oxygen species, increased nitric oxide levels, decreased ATP production, and activation of mitochondrial-dependent apoptosis pathways, including caspase-9 and caspase-3 cleavage, concomitant with an increased BAX/BCL-XL ratio. In contrast, hiPSC-derived NSCs maintain viability by upregulating glycolytic activity, evidenced by increased GLUT-1 expression and hexokinase activity, suggesting a metabolic adaptation that supports resistance to mitochondrial impairment. Notably, the antioxidant N-acetyl-L-cysteine partially restored mitochondrial membrane potential and metabolism in hiPSC-derived NSCs, but failed to protect neurons, highlighting cell-type-specific sensitivity. Alterations in mitochondrial dynamics, particularly decreased OPA-1 and MFN-2 protein expression in neurons, further support a disruption in mitochondrial structure and homeostasis. Our research highlights the translational potential of hiPSC-derived neural models as a powerful platform for unravelling the mechanisms of neurotoxicity induced by PQ and other chemicals associated with Parkinson's disease risk, as well as for uncovering unique cellular responses to oxidative mitochondrial stress. These findings offer critical insights into neuronal vulnerability during early development and provide a foundation for targeted interventions to preserve mitochondrial integrity in neurodegenerative contexts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


