苯酚和醇酯交换法制备磷酸盐桥接光亲和标记探针。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报告了一种温和而通用的方法,用于将光亲和标签安装到酚类和酒精类生物活性分子上。设计了4-硝基苯磷酸和磷酰胺基磷酸基给体,分别对含酚和含醇药物进行选择性修饰。该反应具有广泛的底物范围、良好的官能团耐受性和与竞争性亲核试剂的相容性。凝胶内荧光成像表明,由此产生的磷酸盐桥接光亲和探针是稳定的,并有效地标记细胞蛋白质,强调了它们在化学蛋白质组学应用中的实用性,如目标识别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
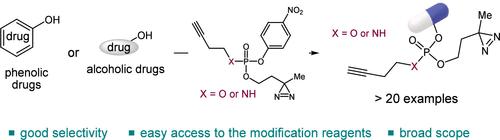
Synthesis of a Phosphate-Bridged Photoaffinity Labeling Probe via Transesterification of Phenols and Alcohols
We report a mild and versatile method for installing photoaffinity tags onto phenolic and alcoholic bioactive molecules. 4-Nitrophenyl phosphate and phosphoramidate based phosphoryl donors were designed to selectively modify phenol- and alcohol-containing drugs, respectively. The reactions proceeded with a broad substrate scope, good functional group tolerance, and compatibility with competing nucleophiles. In-gel fluorescence imaging demonstrated that the resulting phosphate-bridged photoaffinity probes are stable and effectively label cellular proteins, underscoring their utility for chemoproteomic applications, such as target identification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


