rho相关激酶1 (ROCK1)的抑制通过减弱线粒体裂变促进人造血干细胞的扩增
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
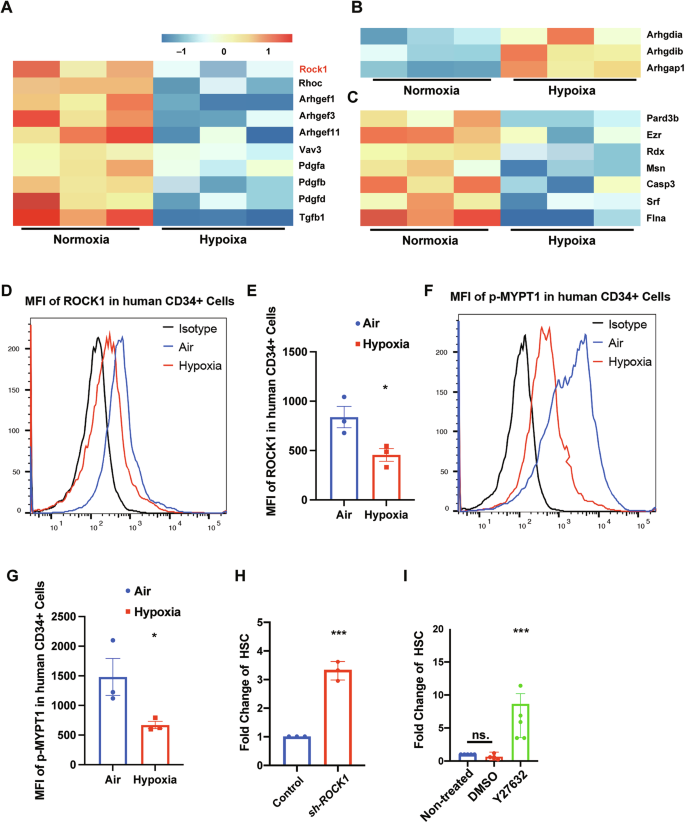
造血干细胞(hsc)的体外扩增受到线粒体应激诱导的干性丧失的限制。为了确定类似于缺氧骨髓生态位的保护机制,我们对缺氧收集的造血干细胞进行了单细胞转录组学研究,发现rho相关激酶1 (ROCK1)显著下调。这一观察结果表明ROCK1可能是HSC稳态的潜在调节因子。我们用shRNA或Y27632通过基因或药理学抑制ROCK1来验证这一点,这可以减少线粒体活性氧、线粒体质量和膜电位,同时促进表型hsc的扩增(Lin⁻CD34⁺CD38⁻CD45RA⁻CD49f⁺CD90⁺)。从机制上讲,ROCK1抑制通过降低p-DRP1(Ser616)和增加p-DRP1(Ser637)来减弱drp1介导的线粒体分裂,从而减少线粒体断裂。此外,ROCK1抑制上调BCL2表达,降低活性Caspase-3水平,表明细胞凋亡受到抑制。限制性稀释移植显示ROCK1抑制后功能性HSC频率增加4倍,并增强了继发受体的长期植入。我们的研究结果确定了ROCK1是造血干细胞线粒体动力学的关键调节因子,并为靶向ROCK1增强造血干细胞功能性扩增提供了机制基础,为通过模拟体外缺氧生态位信号改善造血干细胞移植结果提供了有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Suppression of Rho-associated kinase 1 (ROCK1) promotes human hematopoietic stem cell expansion by attenuating mitochondrial fission
Ex vivo expansion of hematopoietic stem cells (HSCs) is limited by mitochondrial stress-induced loss of stemness. To identify protective mechanisms resembling the hypoxic bone marrow niche, we performed single-cell transcriptomics on hypoxia-collected HSCs, revealing significant downregulation of Rho-associated kinase 1 (ROCK1). This observation suggested ROCK1 as a potential regulator of HSC homeostasis. We tested this by genetically or pharmacologically inhibiting ROCK1 with shRNA or Y27632, which reduced mitochondrial reactive oxygen species, mitochondrial mass, and membrane potential while promoting expansion of phenotypic HSCs (Lin⁻CD34⁺CD38⁻CD45RA⁻CD49f⁺CD90⁺). Mechanistically, ROCK1 inhibition attenuated DRP1-mediated mitochondrial fission by decreasing p-DRP1(Ser616) and increasing p-DRP1(Ser637), thereby reducing mitochondrial fragmentation. Additionally, ROCK1 inhibition elevated BCL2 expression and reduced active Caspase-3 levels, indicating suppressed apoptosis. Limiting dilution transplants demonstrated a fourfold increase in functional HSC frequency following ROCK1 inhibition, with enhanced long-term engraftment in secondary recipients. Our findings identify ROCK1 as a critical regulator of mitochondrial dynamics in HSCs and provide a mechanistic basis for targeting ROCK1 to enhance functional HSC expansion, offering a promising strategy to improve outcomes in HSC transplantation by mimicking hypoxic niche signals ex vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


