单核细胞USP7-p65轴介导髓核免疫原性的免疫应答。
IF 3.2
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
椎间盘髓核(NP)是一种免疫特权组织。在椎间盘退变(IDD)期间,这种免疫特权受到损害,导致NP成分暴露于外周免疫系统,这反过来激活单核细胞并引发免疫反应。在这项研究中,我们证明单核细胞通过激活损伤相关分子模式(DAMPs)来响应NP免疫原性,从而在NP组织中启动持续的NF-κ b介导的炎症反应,并最终在NP细胞内驱动炎症和氧化应激的恶性循环。从机制上讲,np衍生的免疫原性刺激诱导单核细胞活化,并伴随去泛素酶USP7的表达增加和核易位。USP7通过去泛素化依赖机制促进NF-κB亚基p65的积累和核易位,导致TNF-α、HMGB1和IL-1β的转录增强。这些与damp相关的细胞因子进一步刺激NP细胞,导致HMGB1、TNF-α、COX-2、IL-1β和ROS的上调,并伴随抗氧化酶sod2的降低,共同放大NP微环境中的炎症和氧化应激。双荧光素酶报告基因检测和染色质免疫沉淀(ChIP)-qPCR表明,单核细胞中USP7的敲低可显著降低p65与TNF-α、HMGB1和IL-1β启动子区域的结合,从而减轻NP细胞中的下游炎症和氧化应激反应。总之,这些发现揭示了IDD背后的一种新的免疫炎症机制,并强调了单核细胞中usp7介导的途径是调节椎间盘退变的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
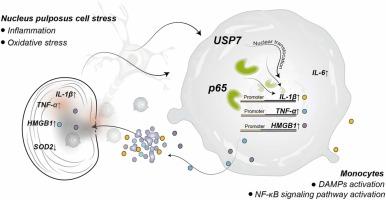
Monocyte USP7-p65 axis mediates immune responses to the immunogenicity of nucleus pulposus
The nucleus pulposus (NP) of the intervertebral disc is an immune-privileged tissue. During intervertebral disc degeneration (IDD), this immune privilege is compromised, resulting in the exposure of NP components to the peripheral immune system, which in turn activates monocytes and elicits an immune response. In this study, we demonstrate that monocytes respond to NP immunogenicity by activating damage-associated molecular patterns (DAMPs), thereby initiating a sustained NF-κB–mediated inflammatory response in NP tissue and ultimately driving a vicious cycle of inflammation and oxidative stress within NP cells. Mechanistically, NP-derived immunogenic stimulation induces monocyte activation, accompanied by increased expression and nuclear translocation of the deubiquitinase USP7. USP7 promotes the accumulation and nuclear translocation of the NF-κB subunit p65 via a deubiquitination-dependent mechanism, leading to enhanced transcription of TNF-α, HMGB1, and IL-1β. These DAMP-associated cytokines further stimulate NP cells, resulting in upregulation of HMGB1, TNF-α, COX-2, IL-1β, and reactive oxygen species (ROS), along with a concomitant decrease in the antioxidant enzyme SOD2—collectively amplifying inflammation and oxidative stress within the NP microenvironment. Dual-luciferase reporter assays and chromatin immunoprecipitation (ChIP)-qPCR demonstrated that knockdown of USP7 in monocytes significantly reduced p65 binding to the promoter regions of TNF-α, HMGB1, and IL-1β, thereby attenuating the downstream inflammatory and oxidative stress responses in NP cells. Together, these findings uncover a novel immune-inflammatory mechanism underlying IDD and highlight the USP7-mediated pathway in monocytes as a potential therapeutic target for modulating disc degeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Stress & Chaperones
生物-细胞生物学
CiteScore
7.60
自引率
2.60%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Cell Stress and Chaperones is an integrative journal that bridges the gap between laboratory model systems and natural populations. The journal captures the eclectic spirit of the cellular stress response field in a single, concentrated source of current information. Major emphasis is placed on the effects of climate change on individual species in the natural environment and their capacity to adapt. This emphasis expands our focus on stress biology and medicine by linking climate change effects to research on cellular stress responses of animals, micro-organisms and plants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


