靶向tdp -43激活的GRP78/内质网应激轴抑制三阴性乳腺癌进展
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
TAR DNA结合蛋白-43 (TDP-43)是由TARDBP基因编码的高度保守的DNA/RNA结合蛋白,在神经退行性疾病领域获得了广泛的科学关注。值得注意的是,近年来其与恶性肿瘤的关联越来越明显。本研究旨在探讨TDP-43在三阴性乳腺癌(TNBC)中的作用,并阐明其潜在机制。生物信息学分析显示,TDP-43在乳腺癌组织中显著上调。在体外,TDP-43表达升高刺激TNBC细胞增殖和迁移,在体内加速肿瘤进展。TDP-43被鉴定为肿瘤生长的促进因子,其抑制作用导致细胞生长明显受阻。此外,葡萄糖调节蛋白78 (GRP78)的过表达抵消了TDP-43敲低时观察到的对细胞增殖和迁移的抑制作用,证实了TDP-43在乳腺癌细胞增殖和迁移中的关键作用。机制研究表明,TDP-43直接结合GRP78 mRNA,刺激其表达。GRP78是未折叠蛋白反应的主要调控因子,其上调激活了肌醇要求酶1α (IRE1α)途径,加剧了内质网(ER)应激。因此,这一过程增强了TNBC细胞的增殖和迁移,显著促进了肿瘤的进展。在这里,我们证明了TDP-43通过调节乳腺癌中的内质网应激促进细胞增殖和迁移,为TNBC中TDP-43的机制复杂性提供了有价值的见解,并对这种情况下的精确治疗具有潜在的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
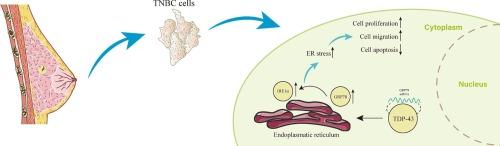
Targeting TDP-43-activated GRP78/endoplasmic reticulum stress axis suppresses triple-negative breast cancer progression
The TAR DNA-binding protein-43 (TDP-43), a highly conserved DNA/RNA binding protein encoded by the TARDBP gene, has garnered extensive scientific attention in the realm of neurodegenerative diseases. Notably, its association with malignant tumors has become increasingly apparent in recent years. This investigation aims to examine the role of TDP-43 in triple-negative breast cancer (TNBC) and to clarify the underlying mechanisms. Bioinformatics analysis showed that TDP-43 was substantially up-regulated in breast cancer tissues. Elevated TDP-43 expression stimulated TNBC cell proliferation and migration in vitro, and accelerated tumor progression in vivo. TDP-43 was identified as a promoter of tumor growth, with its inhibition resulting in a significant impediment to cell growth. Additionally, the overexpression of Glucose-regulated protein 78 (GRP78) counteracted the suppressive effect on cell proliferation and migration observed upon TDP-43 knockdown, affirming TDP-43′s critical role in breast cancer cell proliferation and migration. Mechanistic investigations have demonstrated that TDP-43 binds directly to GRP78 mRNA, stimulating its expression. The up-regulation of GRP78, a master regulator of the unfolded protein response, activates the inositol-requiring enzyme 1 alpha (IRE1α) pathway, exacerbating endoplasmic reticulum (ER) stress. Consequently, this process enhances the proliferation and migration of TNBC cells, contributing significantly to tumor progression. Here, we demonstrate that TDP-43 promotes cell proliferation and migration by regulating ER stress in breast cancer, offering valuable insights into the mechanistic intricacies of TDP-43 in TNBC and holding potential implications for precision treatments in this context.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


