低剂量丙戊酸通过EZH2/H3K27me3信号通路,通过恢复氧化还原稳态和抑制铁蛋白依赖性铁凋亡来改善骨质疏松症。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:丙戊酸(VPA)是一种组蛋白去乙酰化酶抑制剂,对骨稳态具有剂量依赖性。本研究探讨低剂量VPA是否通过靶向氧化应激和铁下垂来预防卵巢切除术(OVX)诱导的骨质疏松症。方法:OVX大鼠接受低剂量(100 mg/kg/d)或高剂量(300 mg/kg/d) VPA治疗12周。显微ct分析骨显微结构。通过测定MDA、SOD、GSH和Fe2+来评估全身氧化还原状态。检测铁下垂标志物(GPX4、ACSL4、FTH1、NCOA4)。在VPA (0.5 ~ 3mm)预处理的MC3T3-E1细胞中,用erastin诱导铁凋亡。进一步评估EZH2/H3K27me3通路和破骨细胞发生。结果:OVX诱导骨丢失、氧化应激(MDA/Fe2+升高,SOD/GSH降低)和铁凋亡激活(ACSL4/NCOA4升高,GPX4/FTH1降低)。低剂量VPA逆转了这些变化,改善了骨密度和微结构,减少了骨吸收。大剂量VPA无保护作用。在体外,1mm VPA可减轻骨质疏松蛋白诱导的脂质过氧化、线粒体损伤和铁下垂。从机制上讲,VPA激活EZH2/H3K27me3信号,增强NCOA4启动子处H3K27me3的富集,从而抑制铁蛋白自噬和铁凋亡。VPA还能抑制rankl诱导的破骨细胞分化。结论:低剂量VPA通过恢复氧化还原稳态、通过活化EZH2/H3K27me3表观遗传抑制ncoa4介导的铁蛋白吞噬、抑制破骨细胞生成等途径改善骨质疏松症。这些发现确定了低剂量VPA是一种多方面的抗骨质疏松剂,并强调了EZH2/H3K27me3/NCOA4轴在骨氧化还原生物学中的关键调控途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
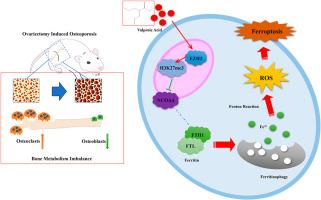
Low-dose valproic acid ameliorates osteoporosis by restoring redox homeostasis and suppressing ferritinophagy-dependent ferroptosis via EZH2/H3K27me3 signaling
Background
Valproic acid (VPA), a histone deacetylase inhibitor, exhibits dose-dependent effects on bone homeostasis. This study investigates whether low-dose VPA protects against ovariectomy (OVX)-induced osteoporosis by targeting oxidative stress and ferroptosis.
Methods
OVX rats received low- (100 mg/kg/d) or high-dose (300 mg/kg/d) VPA for 12 weeks. Bone microstructure was analyzed by micro-CT. Systemic redox status was evaluated by measuring MDA, SOD, GSH, and Fe2+. Ferroptosis markers (GPX4, ACSL4, FTH1, NCOA4) were examined. In MC3T3-E1 cells pretreated with VPA (0.5–3 mM), erastin was used to induce ferroptosis. The EZH2/H3K27me3 pathway and osteoclastogenesis were further assessed.
Results
OVX induced bone loss, oxidative stress (elevated MDA/Fe2+, decreased SOD/GSH), and ferroptosis activation (increased ACSL4/NCOA4, decreased GPX4/FTH1). Low-dose VPA reversed these changes, improved bone density and microarchitecture, and reduced bone resorption. High-dose VPA showed no protective effects. In vitro, 1 mM VPA attenuated erastin-induced lipid peroxidation, mitochondrial damage, and ferroptosis. Mechanistically, VPA activated EZH2/H3K27me3 signaling, enhancing H3K27me3 enrichment at the NCOA4 promoter to suppress ferritinophagy and ferroptosis. VPA also inhibited RANKL-induced osteoclast differentiation.
Conclusion
Low-dose VPA ameliorates osteoporosis by restoring redox homeostasis, epigenetically inhibiting NCOA4-mediated ferritinophagy via EZH2/H3K27me3 activation, and suppressing osteoclastogenesis. These findings identify low-dose VPA as a multifaceted anti-osteoporotic agent and highlight the EZH2/H3K27me3/NCOA4 axis as a pivotal regulatory pathway in bone redox biology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


