Sirt1通过Hif-1α去乙酰化介导的bnip3依赖性线粒体自噬抑制来减轻坏死性小肠结肠炎。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
坏死性小肠结肠炎(NEC)是一种危及生命的新生儿疾病,涉及线粒体功能障碍,其调节尚不清楚。本研究在NEC发病机制中发现了一个新的Sir1/Hif-1α调控轴。我们证明,在NEC中Sirt1下调导致Hif-1α超乙酰化,导致bnip3介导的线粒体自噬激活和肠上皮损伤。通过临床样本和实验模型,我们发现Sirt1下调与线粒体功能障碍和屏障破坏相关。SRT1720激活Sirt1通过Hif-1α去乙酰化和随后的线粒体自噬抑制有效地减弱NEC进展。重要的是,我们提供了Sirt1直接调节肠上皮细胞中Hif-1α乙酰化状态的第一个证据,建立了一种将蛋白质乙酰化与NEC线粒体质量控制联系起来的新的分子机制。这些发现表明Sirt1是肠道稳态的主要调节因子,并强调Sirt1激活是一种有希望的NEC治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
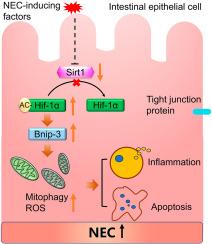
Sirt1 attenuates necrotizing enterocolitis via Hif-1α deacetylation-mediated suppression of Bnip3-Dependent mitophagy
Necrotizing enterocolitis (NEC), a life-threatening neonatal disease, involves mitochondrial dysfunction whose regulation remains unclear. This study identifies a novel Sir1/Hif-1α regulatory axis in NEC pathogenesis. We demonstrate that Sirt1 downregulation in NEC leads to Hif-1α hyperacetylation, resulting in Bnip3-mediated mitophagy activation and intestinal epithelial injury. Using clinical samples and experimental models, we show that Sirt1 downregulation correlates with mitochondrial dysfunction and intestinal barrier disruption. Pharmacological Sirt1 activation by SRT1720 effectively attenuated NEC progression through Hif-1α deacetylation and subsequent mitophagy inhibition. Importantly, we provide the first evidence that Sirt1 directly regulates Hif-1α acetylation status in intestinal epithelial cells, establishing a new molecular mechanism linking protein acetylation to mitochondrial quality control in NEC. These findings reveal Sirt1 as a master regulator of intestinal homeostasis and highlight Sirt1 activation as a promising therapeutic approach for NEC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


