活性氧和炎性巨噬细胞增加对主动脉瓣钙化发展的贡献。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
主动脉瓣钙化病(CAVD)是一种活动性心血管疾病,是主动脉瓣狭窄最常见的原因。患冠心病的风险随着年龄的增长而增加。本研究建立了主动脉瓣纤维层的三维(3D)模型,以评估活性氧(ROS)存在下单核/巨噬细胞在CAVD发展中的作用。3D模型包括猪主动脉瓣间质细胞(PAVIC)、猪主动脉瓣内皮细胞(PAVECs)和分化为m1样巨噬细胞的THP-1单核细胞。采用浓度为100 μM的过氧化氢(H2O2)诱导ROS,模拟病理生理ROS条件,在维持细胞活力的同时促进钙化。在体外3D模型中,长时间暴露于H2O2(长达14天)导致钙化。H2O2预处理后引入THP-1单核细胞或m1样巨噬细胞加速钙化过程。与对照和共培养模型相比,m1 -三培养模型形成了钙和磷酸盐含量增加的结节。除m1 -三培养模型外,H2O2处理抑制水凝胶收缩,表明ROS与细胞收缩有关。这些发现为CAVD的发病机制提供了信息,证明了单核/巨噬细胞和ROS在主动脉瓣纤维层钙化中的关键作用。这个3D模型为进一步研究cavd特异性治疗策略提供了一个强大的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
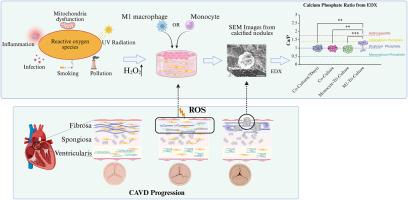
The contributions of increased reactive oxygen species and inflammatory macrophages to the development of aortic valve calcification
Calcific aortic valve disease (CAVD), an active cardiovascular disease, is the most common cause of aortic valve stenosis. The risk of developing CAVD increases with age. In this study, a three-dimensional (3D) model of the aortic valve fibrosa layer was created to evaluate the role of monocytes/macrophages in the development of CAVD in the presence of reactive oxygen species (ROS). The 3D model includes porcine aortic valve interstitial cells (PAVIC), porcine aortic valve endothelial cells (PAVECs), and THP-1 monocytes that have differentiated into M1-like macrophages. Hydrogen peroxide (H2O2) was used to induce ROS at a concentration of 100 μM to mimic pathophysiological ROS conditions and promote calcification while maintaining cell viability. Prolonged exposure to H2O2 (up to 14 days) caused calcification in the in vitro 3D model. The introduction of THP-1 monocytes or M1-like macrophages after pretreatment with H2O2 accelerated the calcification process. The M1-tri-culture model formed nodules with increased calcium and phosphate when compared with controls and co-culture models. Except in the M1-tri-culture model, H2O2 treatment inhibited hydrogel contraction, indicating a connection between ROS and cellular contraction. These findings provide information on the pathogenesis of CAVD, demonstrating the crucial role of monocytes/macrophages and ROS in the calcification of the aortic valve fibrosa layer. This 3D model offers a robust platform for additional study on CAVD-specific therapeutic strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


