KDM5C:一种双刃剑表观遗传调控因子在癌症生物学环境依赖性作用和治疗意义。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
赖氨酸特异性去甲基化酶5C (KDM5C是一种组蛋白去甲基化酶)是H3K4甲基化的关键表观遗传调节剂,在癌症进展中具有环境依赖性作用。在透明细胞肾细胞癌(ccRCC)中,它通过维持基因组稳定性和抑制HIF信号传导而发挥肿瘤抑制作用,同时,它通过促进上皮-间质转化(EMT)、代谢重编程和化疗耐药,在前列腺癌、乳腺癌和肝细胞癌中发挥致癌驱动作用。KDM5C通过与关键调节因子(ARX、Smad3和HIF1α)的相互作用和染色质景观的重塑来协调这些相反的功能,以增强肿瘤的可塑性。治疗耐药机制强调了其临床意义,包括CD44在胰腺癌中的协同作用和HPV e6介导的lncRNA在宫颈癌中的激活。尽管临床前研究表明KDM5C抑制剂可以克服药物耐受性并抑制肿瘤发生,但在实现亚型特异性和减少脱靶效应方面仍然存在挑战。除肿瘤学外,KDM5C失调还可导致Claes-Jensen综合征和神经发育障碍,这反映了它的多效性。未来的方向应该整合单细胞组学和靶向抑制剂开发,以阐明微环境相互作用和实现精确治疗。这种合成将KDM5C定位为疾病发病机制中的关键表观遗传联系,提供了依赖于环境的治疗机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
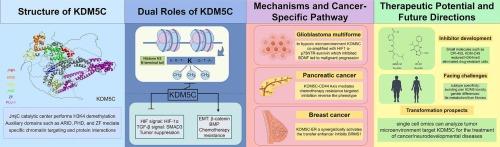
KDM5C: A dual-edged epigenetic regulator in cancer biology-context-dependent roles and therapeutic implications
Lysine-specific demethylase 5C (KDM5C is a histone demethylase that) serves as a critical epigenetic regulator of H3K4 methylation, with context-dependent roles in cancer progression. While functioning as a tumor suppressor in clear-cell renal cell carcinoma (ccRCC) by maintaining genomic stability and inhibiting HIF signaling, it acts as an oncogenic driver in prostate, breast, and hepatocellular carcinomas by promoting epithelial–mesenchymal transition (EMT), metabolic reprogramming, and chemoresistance. KDM5C orchestrates these opposing functions via interactions with key regulators (ARX, Smad3, and HIF1α) and remodeling of chromatin landscapes to enhance tumor plasticity. Its clinical significance is underscored by therapeutic resistance mechanisms, including CD44 cooperation in pancreatic cancer and HPV E6-mediated lncRNA activation in cervical cancer. Although preclinical studies demonstrate that KDM5C inhibitors can overcome drug tolerance and suppress tumorigenesis, challenges persist in achieving subtype specificity and reducing off-target effects. Beyond oncology, KDM5C dysregulation contributes to Claes-Jensen syndrome and neurodevelopmental disorders, reflecting its pleiotropic functions. Future directions should integrate single-cell omics and targeted inhibitor development to elucidate microenvironmental interactions and enable precision therapies. This synthesis positions KDM5C as a pivotal epigenetic nexus in disease pathogenesis, offering context-dependent therapeutic opportunities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


